The Use of Pro-Angiogenic and/or Pro-Hypoxic miRNAs as Tools to Monitor Patients with Diffuse Gliomas
- PMID: 35682718
- PMCID: PMC9181142
- DOI: 10.3390/ijms23116042
The Use of Pro-Angiogenic and/or Pro-Hypoxic miRNAs as Tools to Monitor Patients with Diffuse Gliomas
Abstract
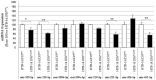
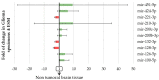
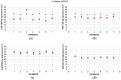
IDH (isocitrate dehydrogenase) mutation, hypoxia, and neo-angiogenesis, three hallmarks of diffuse gliomas, modulate the expression of small non-coding RNAs (miRNA). In this paper, we tested whether pro-angiogenic and/or pro-hypoxic miRNAs could be used to monitor patients with glioma. The miRNAs were extracted from tumoral surgical specimens embedded in the paraffin of 97 patients with diffuse gliomas and, for 7 patients, from a blood sample too. The expression of 10 pro-angiogenic and/or pro-hypoxic miRNAs was assayed by qRT-PCR and normalized to the miRNA expression of non-tumoral brain tissues. We confirmed in vitro that IDH in hypoxia (1% O2, 24 h) alters pro-angiogenic and/or pro-hypoxic miRNA expression in HBT-14 (U-87 MG) cells. Then, we reported that the expression of these miRNAs is (i) strongly affected in patients with glioma compared to that in a non-tumoral brain; (ii) correlated with the histology/grade of glioma according to the 2016 WHO classification; and (iii) predicts the overall and/or progression-free survival of patients with glioma in univariate but not in a multivariate analysis after adjusting for sex, age at diagnosis, and WHO classification. Finally, the expression of miRNAs was found to be the same between the plasma and glial tumor of the same patient. This study highlights a panel of seven pro-angiogenic and/or pro-hypoxic miRNAs as a potential tool for monitoring patients with glioma.
Keywords: angiogenesis; glioma; hypoxia; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Use of telomerase promoter mutations to mark specific molecular subsets with reciprocal clinical behavior in IDH mutant and IDH wild-type diffuse gliomas.J Neurosurg. 2018 Apr;128(4):1102-1114. doi: 10.3171/2016.11.JNS16973. Epub 2017 Jun 16. J Neurosurg. 2018. PMID: 28621624
-
IDH1R¹³²H decreases the proliferation of U87 glioma cells through upregulation of microRNA-128a.Mol Med Rep. 2015 Nov;12(5):6695-701. doi: 10.3892/mmr.2015.4241. Epub 2015 Aug 24. Mol Med Rep. 2015. PMID: 26324126 Free PMC article.
-
A nuclear transport-related gene signature combined with IDH mutation and 1p/19q codeletion better predicts the prognosis of glioma patients.BMC Cancer. 2020 Nov 9;20(1):1072. doi: 10.1186/s12885-020-07552-3. BMC Cancer. 2020. PMID: 33167941 Free PMC article.
-
Integrated diagnostics of diffuse astrocytic and oligodendroglial tumors.Pathologe. 2019 Jun;40(Suppl 1):9-17. doi: 10.1007/s00292-019-0581-8. Pathologe. 2019. PMID: 31025086 Review. English.
-
Clinical impact of revisions to the WHO classification of diffuse gliomas and associated future problems.Int J Clin Oncol. 2020 Jun;25(6):1004-1009. doi: 10.1007/s10147-020-01628-7. Epub 2020 Feb 4. Int J Clin Oncol. 2020. PMID: 32020379 Review.
Cited by
-
Different Approaches to Study Molecular Blueprint and Biological Behavior of Brain Tumors: Editorial to the Special Issue "Advances in Molecular Genetics of Brain Tumors".Int J Mol Sci. 2023 Jan 4;24(2):948. doi: 10.3390/ijms24020948. Int J Mol Sci. 2023. PMID: 36674461 Free PMC article.
References
-
- Torrisi F., Alberghina C., D’Aprile S., Pavone A.M., Longhitano L., Giallongo S., Tibullo D., Di Rosa M., Zappalà A., Cammarata F.P., et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines. 2022;10:806. doi: 10.3390/biomedicines10040806. - DOI - PMC - PubMed
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

