Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells
- PMID: 35682723
- PMCID: PMC9181642
- DOI: 10.3390/ijms23116048
Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells
Abstract
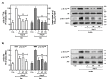
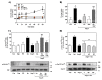
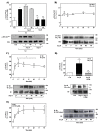
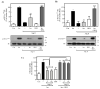
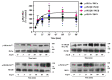
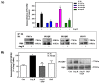
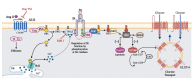
Angiotensin II (Ang II) is a critical regulator of insulin signaling in the cardiovascular system and metabolic tissues. However, in adipose cells, the regulatory role of Ang II on insulin actions remains to be elucidated. The effect of Ang II on insulin-induced insulin receptor (IR) phosphorylation, Akt activation, and glucose uptake was examined in 3T3-L1 adipocytes. In these cells, Ang II specifically inhibited insulin-stimulated IR and insulin receptor substrate-1 (IRS-1) tyrosine-phosphorylation, Akt activation, and glucose uptake in a time-dependent manner. These inhibitory actions were associated with increased phosphorylation of the IR at serine residues. Interestingly, Ang II-induced serine-phosphorylation of IRS was not detected, suggesting that Ang II-induced desensitization begins from IR regulation itself. PKC inhibition by BIM I restored the inhibitory effect of Ang II on insulin actions. We also found that Ang II promoted activation of several PKC isoforms, including PKCα/βI/βII/δ, and its association with the IR, particularly PKCβII, showed the highest interaction. Finally, we also found a similar regulatory effect of Ang II in isolated adipocytes, where insulin-induced Akt phosphorylation was inhibited by Ang II, an effect that was prevented by PKC inhibitors. These results suggest that Ang II may lead to insulin resistance through PKC activation in adipocytes.
Keywords: adipose cells; angiotensin II; insulin receptor; insulin resistance; protein kinase C; serine-phosphorylation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells.Exp Cell Res. 2005 Aug 15;308(2):291-9. doi: 10.1016/j.yexcr.2005.04.028. Exp Cell Res. 2005. PMID: 15921682
-
Angiotensin-induced EGF receptor transactivation inhibits insulin signaling in C9 hepatic cells.Biochem Pharmacol. 2010 Mar 1;79(5):733-45. doi: 10.1016/j.bcp.2009.10.014. Epub 2009 Oct 29. Biochem Pharmacol. 2010. PMID: 19879250 Free PMC article.
-
Angiotensin-(1 7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors.Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1154-63. doi: 10.1152/ajpheart.01395.2006. Epub 2007 May 11. Am J Physiol Heart Circ Physiol. 2007. PMID: 17496209
-
Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms.PLoS One. 2010 Apr 7;5(4):e10070. doi: 10.1371/journal.pone.0010070. PLoS One. 2010. PMID: 20383279 Free PMC article.
-
Insulin-induced Akt activation is inhibited by angiotensin II in the vasculature through protein kinase C-alpha.Hypertension. 2003 Mar;41(3 Pt 2):775-80. doi: 10.1161/01.HYP.0000051891.90321.12. Epub 2003 Jan 13. Hypertension. 2003. PMID: 12623995
Cited by
-
Cellular Senescence and Extracellular Vesicles in the Pathogenesis and Treatment of Obesity-A Narrative Review.Int J Mol Sci. 2024 Jul 20;25(14):7943. doi: 10.3390/ijms25147943. Int J Mol Sci. 2024. PMID: 39063184 Free PMC article. Review.
-
Proton pump inhibitors use and risk of type 2 diabetes mellitus: correlation analysis, prediction model construction, and key genes identification.Front Pharmacol. 2025 Apr 29;16:1580090. doi: 10.3389/fphar.2025.1580090. eCollection 2025. Front Pharmacol. 2025. PMID: 40365304 Free PMC article.
-
Effects of AGT and AGTR1 Genetic Polymorphisms and Changes in Blood Pressure Over a Five-Year Follow-Up.Risk Manag Healthc Policy. 2023 Dec 28;16:2931-2942. doi: 10.2147/RMHP.S442983. eCollection 2023. Risk Manag Healthc Policy. 2023. PMID: 38164294 Free PMC article.
-
The PI3K/Akt Pathway in Meta-Inflammation.Int J Mol Sci. 2022 Dec 5;23(23):15330. doi: 10.3390/ijms232315330. Int J Mol Sci. 2022. PMID: 36499659 Free PMC article. Review.
-
Skeletal muscle atrophy and dysfunction in obesity and type-2 diabetes mellitus: Myocellular mechanisms involved.Rev Endocr Metab Disord. 2025 Mar 10. doi: 10.1007/s11154-025-09954-9. Online ahead of print. Rev Endocr Metab Disord. 2025. PMID: 40064750 Review.
References
-
- Gutierrez-Rodelo C., Roura-Guiberna A., Olivares-Reyes J.A. Molecular mechanisms of insulin resistance: An update. Gac. Med. Mex. 2017;153:197–209. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

