Deletion of the Autism-Associated Protein SHANK3 Abolishes Structural Synaptic Plasticity after Brain Trauma
- PMID: 35682760
- PMCID: PMC9181590
- DOI: 10.3390/ijms23116081
Deletion of the Autism-Associated Protein SHANK3 Abolishes Structural Synaptic Plasticity after Brain Trauma
Abstract
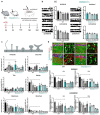
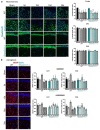
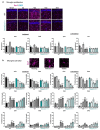
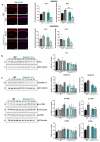
Autism spectrum disorders (ASDs) are characterized by repetitive behaviors and impairments of sociability and communication. About 1% of ASD cases are caused by mutations of SHANK3, a major scaffolding protein of the postsynaptic density. We studied the role of SHANK3 in plastic changes of excitatory synapses within the central nervous system by employing mild traumatic brain injury (mTBI) in WT and Shank3 knockout mice. In WT mice, mTBI triggered ipsi- and contralateral loss of hippocampal dendritic spines and excitatory synapses with a partial recovery over time. In contrast, no significant synaptic alterations were detected in Shank3∆11-/- mice, which showed fewer dendritic spines and excitatory synapses at baseline. In line, mTBI induced the upregulation of synaptic plasticity-related proteins Arc and p-cofilin only in WT mice. Interestingly, microglia proliferation was observed in WT mice after mTBI but not in Shank3∆11-/- mice. Finally, we detected TBI-induced increased fear memory at the behavioral level, whereas in Shank3∆11-/- animals, the already-enhanced fear memory levels increased only slightly after mTBI. Our data show the lack of structural synaptic plasticity in Shank3 knockout mice that might explain at least in part the rigidity of behaviors, problems in adjusting to new situations and cognitive deficits seen in ASDs.
Keywords: ASD; SHANK3; TBI; plasticity; synapses; trauma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Trans-synaptic Association of Vesicular Zinc Transporter 3 and Shank3 Supports Synapse-Specific Dendritic Spine Structure and Function in the Mouse Auditory Cortex.J Neurosci. 2024 Jul 10;44(28):e0619242024. doi: 10.1523/JNEUROSCI.0619-24.2024. J Neurosci. 2024. PMID: 38830758 Free PMC article.
-
Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium.Mol Psychiatry. 2024 Mar;29(3):704-717. doi: 10.1038/s41380-023-02362-y. Epub 2023 Dec 20. Mol Psychiatry. 2024. PMID: 38123724 Free PMC article.
-
Behavioral decline in Shank3Δex4-22 mice during early adulthood parallels cerebellar granule cell glutamatergic synaptic changes.Mol Autism. 2024 Dec 4;15(1):52. doi: 10.1186/s13229-024-00628-y. Mol Autism. 2024. PMID: 39633421 Free PMC article.
-
Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications.J Neurodev Disord. 2021 Nov 16;13(1):55. doi: 10.1186/s11689-021-09397-8. J Neurodev Disord. 2021. PMID: 34784886 Free PMC article. Review.
-
SHANK3 as an autism spectrum disorder-associated gene.Brain Dev. 2013 Feb;35(2):106-10. doi: 10.1016/j.braindev.2012.05.013. Epub 2012 Jun 29. Brain Dev. 2013. PMID: 22749736 Review.
Cited by
-
Microglia and Astrocytes Responses Contribute to Alleviating Inflammatory Damage by Repetitive Transcranial Magnetic Stimulation in Rats with Traumatic Brain Injury.Neurochem Res. 2024 Sep;49(9):2636-2651. doi: 10.1007/s11064-024-04197-7. Epub 2024 Jun 23. Neurochem Res. 2024. PMID: 38909329
-
Expression profiles of the autism-related SHANK proteins in the human brain.BMC Biol. 2023 Nov 13;21(1):254. doi: 10.1186/s12915-023-01712-0. BMC Biol. 2023. PMID: 37953224 Free PMC article.
-
Non-Genetic Modifiers of Synaptic Plasticity and Neurotransmission in the Central Nervous System (CNS) in Health and Disease.Int J Mol Sci. 2022 Oct 12;23(20):12135. doi: 10.3390/ijms232012135. Int J Mol Sci. 2022. PMID: 36292989 Free PMC article.
-
Development of sex- and genotype-specific behavioral phenotypes in a Shank3 mouse model for neurodevelopmental disorders.Front Behav Neurosci. 2023 Jan 9;16:1051175. doi: 10.3389/fnbeh.2022.1051175. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36699652 Free PMC article.
-
Autism Spectrum Disorder: Brain Areas Involved, Neurobiological Mechanisms, Diagnoses and Therapies.Int J Mol Sci. 2024 Feb 19;25(4):2423. doi: 10.3390/ijms25042423. Int J Mol Sci. 2024. PMID: 38397100 Free PMC article. Review.
References
-
- Bralten J., van Hulzen K.J., Martens M.B., Galesloot T.E., Arias Vasquez A., Kiemeney L.A., Buitelaar J.K., Muntjewerff J.W., Franke B., Poelmans G. Autism spectrum disorders and autistic traits share genetics and biology. Mol. Psychiatry. 2018;23:1205–1212. doi: 10.1038/mp.2017.98. - DOI - PMC - PubMed
-
- Boccuto L., Lauri M., Sarasua S.M., Skinner C.D., Buccella D., Dwivedi A., Orteschi D., Collins J.S., Zollino M., Visconti P., et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet. 2013;21:310–316. doi: 10.1038/ejhg.2012.175. - DOI - PMC - PubMed
-
- Durand C.M., Betancur C., Boeckers T.M., Bockmann J., Chaste P., Fauchereau F., Nygren G., Rastam M., Gillberg I.C., Anckarsater H., et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007;39:25–27. doi: 10.1038/ng1933. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

