Neu3 Sialidase Activates the RISK Cardioprotective Signaling Pathway during Ischemia and Reperfusion Injury (IRI)
- PMID: 35682772
- PMCID: PMC9181429
- DOI: 10.3390/ijms23116090
Neu3 Sialidase Activates the RISK Cardioprotective Signaling Pathway during Ischemia and Reperfusion Injury (IRI)
Abstract
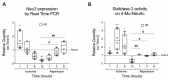
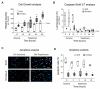
Coronary reperfusion strategies are life-saving approaches to restore blood flow to cardiac tissue after acute myocardial infarction (AMI). However, the sudden restoration of normal blood flow leads to ischemia and reperfusion injury (IRI), which results in cardiomyoblast death, irreversible tissue degeneration, and heart failure. The molecular mechanism of IRI is not fully understood, and there are no effective cardioprotective strategies to prevent it. In this study, we show that activation of sialidase-3, a glycohydrolytic enzyme that cleaves sialic acid residues from glycoconjugates, is cardioprotective by triggering RISK pro-survival signaling pathways. We found that overexpression of Neu3 significantly increased cardiomyoblast resistance to IRI through activation of HIF-1α and Akt/Erk signaling pathways. This raises the possibility of using Sialidase-3 activation as a cardioprotective reperfusion strategy after myocardial infarction.
Keywords: Neu3; cardioprotective strategies; gangliosides; ischemia and reperfusion injury; myocardial infarction; sialidase-3.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
-
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- Roule V., Ardouin P., Blanchart K., Lemaitre A., Wain-Hobson J., Legallois D., Alexandre J., Sabatier R., Milliez P., Beygui F. Prehospital fibrinolysis versus primary percutaneous coronary intervention in ST-elevation myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Crit. Care. 2016;20:359. doi: 10.1186/s13054-016-1530-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

