Rlip76: An Unexplored Player in Neurodegeneration and Alzheimer's Disease?
- PMID: 35682775
- PMCID: PMC9181721
- DOI: 10.3390/ijms23116098
Rlip76: An Unexplored Player in Neurodegeneration and Alzheimer's Disease?
Abstract
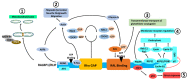
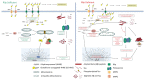
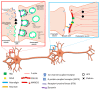
Alzheimer's disease (AD) is a progressive neurodegenerative disorder and is the most common cause of dementia in older people. AD is associated with the loss of synapses, oxidative stress, mitochondrial structural and functional abnormalities, microRNA deregulation, inflammatory responses, neuronal loss, accumulation of amyloid-beta (Aβ) and phosphorylated tau (p-tau). AD occurs in two forms: early onset, familial AD and late-onset, sporadic AD. Causal factors are still unknown for a vast majority of AD patients. Genetic polymorphisms are proposed to contribute to late-onset AD via age-dependent increases in oxidative stress and mitochondrial abnormalities. Recent research from our lab revealed that reduced levels of Rlip76 induce oxidative stress, mitochondrial dysfunction and synaptic damage, leading to molecular and behavioral phenotypes resembling late-onset AD. Rlip76 is a multifunctional 76 kDa protein encoded by the RALBP1 gene, located on chromosome 18. Rlip is a stress-protective ATPase of the mercapturic acid pathway that couples clathrin-dependent endocytosis with the efflux of glutathione-electrophile conjugates. Rlip is evolutionarily highly conserved across species and is ubiquitously expressed in all tissues, including AD-affected brain regions, the cerebral cortex and hippocampus, where highly active neuronal metabolisms render the cells highly susceptible to intracellular oxidative damage. In the current article, we summarize molecular and cellular features of Rlip and how depleted Rlip may exacerbate oxidative stress, mitochondrial dysfunction and synaptic damage in AD. We also discuss the possible role of Rlip in aspects of learning and memory via axonal growth, dendritic remodeling, and receptor regulation. We conclude with a discussion of the potential for the contribution of genetic polymorphisms in Rlip to AD progression and the potential for Rlip-based therapies.
Keywords: Alzheimer’s disease; RALBP1; Rlip; mitochondrial dysfunction; neurodegeneration; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rlip76 in ageing and Alzheimer's disease: Focus on oxidative stress and mitochondrial mechanisms.Ageing Res Rev. 2025 Jan;103:102600. doi: 10.1016/j.arr.2024.102600. Epub 2024 Nov 30. Ageing Res Rev. 2025. PMID: 39617058 Review.
-
RALBP1 in Oxidative Stress and Mitochondrial Dysfunction in Alzheimer's Disease.Cells. 2021 Nov 10;10(11):3113. doi: 10.3390/cells10113113. Cells. 2021. PMID: 34831336 Free PMC article.
-
The role of RLIP76 in oxidative stress and mitochondrial dysfunction: Evidence based on autopsy brains from Alzheimer's disease patients.Biochim Biophys Acta Mol Basis Dis. 2024 Feb;1870(2):166932. doi: 10.1016/j.bbadis.2023.166932. Epub 2023 Nov 4. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 37926360
-
Rlip overexpression reduces oxidative stress and mitochondrial dysfunction in Alzheimer's disease: Mechanistic insights.Biochim Biophys Acta Mol Basis Dis. 2023 Oct;1869(7):166759. doi: 10.1016/j.bbadis.2023.166759. Epub 2023 May 22. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 37225106
-
Molecular Basis of Alzheimer's Disease: Focus on Mitochondria.J Alzheimers Dis. 2019;72(s1):S95-S116. doi: 10.3233/JAD-190048. J Alzheimers Dis. 2019. PMID: 30932888 Review.
Cited by
-
Regulation of cargo exocytosis by a Reps1-Ralbp1-RalA module.Sci Adv. 2023 Feb 22;9(8):eade2540. doi: 10.1126/sciadv.ade2540. Epub 2023 Feb 22. Sci Adv. 2023. PMID: 36812304 Free PMC article.
-
Rlip Reduction Induces Oxidative Stress and Mitochondrial Dysfunction in Mutant Tau-Expressed Immortalized Hippocampal Neurons: Mechanistic Insights.Cells. 2023 Jun 16;12(12):1646. doi: 10.3390/cells12121646. Cells. 2023. PMID: 37371116 Free PMC article.
-
Antibody Assay and Anti-Inflammatory Function Evaluation of Therapeutic Potential of Different Intravenous Immunoglobulins for Alzheimer's Disease.Int J Mol Sci. 2023 Mar 14;24(6):5549. doi: 10.3390/ijms24065549. Int J Mol Sci. 2023. PMID: 36982622 Free PMC article.
-
Causal Relationship Between Basal Metabolic Rate and Alzheimer's Disease: A Bidirectional Two-sample Mendelian Randomization Study.Neurol Ther. 2023 Jun;12(3):763-776. doi: 10.1007/s40120-023-00458-9. Epub 2023 Mar 10. Neurol Ther. 2023. PMID: 36894827 Free PMC article.
References
-
- Yang Y., Sharma A., Sharma R., Patrick B., Singhal S.S., Zimniak P., Awasthi S., Awasthi Y.C. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: Role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J. Biol. Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. - DOI - PubMed
-
- Singhal S.S., Wickramarachchi D., Yadav S., Singhal J., Leake K., Vatsyayan R., Chaudhary P., Lelsani P., Suzuki S., Yang S., et al. Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol. Cancer. 2011;10:16–28. doi: 10.1158/1535-7163.MCT-10-0699. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

