Sophoraflavanone G from Sophora flavescens Ameliorates Allergic Airway Inflammation by Suppressing Th2 Response and Oxidative Stress in a Murine Asthma Model
- PMID: 35682783
- PMCID: PMC9181790
- DOI: 10.3390/ijms23116104
Sophoraflavanone G from Sophora flavescens Ameliorates Allergic Airway Inflammation by Suppressing Th2 Response and Oxidative Stress in a Murine Asthma Model
Abstract
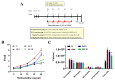
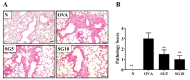
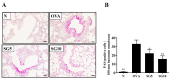
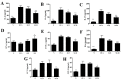
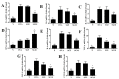
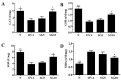
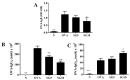
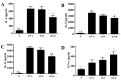
Sophoraflavanone G (SG), isolated from Sophora flavescens, has anti-inflammatory and anti-tumor bioactive properties. We previously showed that SG promotes apoptosis in human breast cancer cells and leukemia cells and reduces the inflammatory response in lipopolysaccharide-stimulated macrophages. We investigated whether SG attenuates airway hyper-responsiveness (AHR) and airway inflammation in asthmatic mice. We also assessed its effects on the anti-inflammatory response in human tracheal epithelial cells. Female BALB/c mice were sensitized with ovalbumin, and asthmatic mice were treated with SG by intraperitoneal injection. We also exposed human bronchial epithelial BEAS-2B cells to different concentrations of SG to evaluate its effects on inflammatory cytokine levels. SG treatment significantly reduced AHR, eosinophil infiltration, goblet cell hyperplasia, and airway inflammation in the lungs of asthmatic mice. In the lungs of ovalbumin-sensitized mice, SG significantly promoted superoxide dismutase and glutathione expression and attenuated malondialdehyde levels. SG also suppressed levels of Th2 cytokines and chemokines in lung and bronchoalveolar lavage samples. In addition, we confirmed that SG decreased pro-inflammatory cytokine, chemokine, and eotaxin expression in inflammatory BEAS-2B cells. Taken together, our data demonstrate that SG shows potential as an immunomodulator that can improve asthma symptoms by decreasing airway-inflammation-related oxidative stress.
Keywords: Th2 cell; airway hyper-responsiveness; airway inflammation; asthma; sophoraflavanone G.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

