APTC-C-SA01: A Novel Bacteriophage Cocktail Targeting Staphylococcus aureus and MRSA Biofilms
- PMID: 35682794
- PMCID: PMC9181636
- DOI: 10.3390/ijms23116116
APTC-C-SA01: A Novel Bacteriophage Cocktail Targeting Staphylococcus aureus and MRSA Biofilms
Abstract
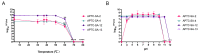
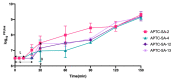
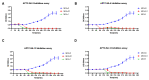
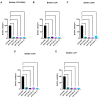
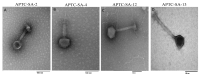
The high infection and mortality rate of methicillin-resistant Staphylococcus aureus (MRSA) necessitates the urgent development of new treatment strategies. Bacteriophages (phages) have several advantages compared to antibiotics for the treatment of multi-drug-resistant bacterial infections, and thus provide a promising alternative to antibiotics. Here, S. aureus phages were isolated from patients and environmental sources. Phages were characterized for stability, morphology and genomic sequence and their bactericidal activity against the biofilm form of methicillin-susceptible Staphylococcus aureus (MSSA) and MRSA was investigated. Four S. aureus phages were isolated and tested against 51 MSSA and MRSA clinical isolates and reference strains. The phages had a broad host range of 82−94% individually and of >98% when combined and could significantly reduce the viability of S. aureus biofilms. The phages had a latent period of ≤20 min and burst size of >11 plaque forming units (PFU)/infected cell. Transmission electron microscopy (TEM) identified phages belonging to the family of Myoviridae. Genomic sequencing indicated the lytic nature of all four phages, with no identified resistance or virulence genes. The 4 phages showed a high complementarity with 49/51 strains (96%) sensitive to at least 2/4 phages tested. Furthermore, the frequency of bacteriophage insensitive mutant (BIM) generation was lower when the phages were combined into the phage cocktail APTC-C-SA01 than for bacteria exposed to each of the phages alone. In conclusion, APTC-C-SA01, containing four lytic S. aureus phages has the potential for further development as a treatment against MSSA and MRSA infections.
Keywords: S. aureus; antimicrobial; bacteriophage; biofilm; phage cocktail.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Aslam M.R., Rizvi T.A., Munawar M.T., Hussain M.A., Samuel T. Fight against Superbugs in a Burn Centre: Are We Doing Enough. PAFMJ. 2021;71:1425–1430. doi: 10.51253/pafmj.v71i4.4307. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

