Thirty Years' History since the Discovery of Pax6: From Central Nervous System Development to Neurodevelopmental Disorders
- PMID: 35682795
- PMCID: PMC9181425
- DOI: 10.3390/ijms23116115
Thirty Years' History since the Discovery of Pax6: From Central Nervous System Development to Neurodevelopmental Disorders
Abstract
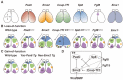
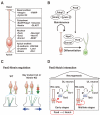
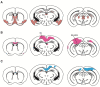
Pax6 is a sequence-specific DNA binding transcription factor that positively and negatively regulates transcription and is expressed in multiple cell types in the developing and adult central nervous system (CNS). As indicated by the morphological and functional abnormalities in spontaneous Pax6 mutant rodents, Pax6 plays pivotal roles in various biological processes in the CNS. At the initial stage of CNS development, Pax6 is responsible for brain patterning along the anteroposterior and dorsoventral axes of the telencephalon. Regarding the anteroposterior axis, Pax6 is expressed inversely to Emx2 and Coup-TF1, and Pax6 mutant mice exhibit a rostral shift, resulting in an alteration of the size of certain cortical areas. Pax6 and its downstream genes play important roles in balancing the proliferation and differentiation of neural stem cells. The Pax6 gene was originally identified in mice and humans 30 years ago via genetic analyses of the eye phenotypes. The human PAX6 gene was discovered in patients who suffer from WAGR syndrome (i.e., Wilms tumor, aniridia, genital ridge defects, mental retardation). Mutations of the human PAX6 gene have also been reported to be associated with autism spectrum disorder (ASD) and intellectual disability. Rodents that lack the Pax6 gene exhibit diverse neural phenotypes, which might lead to a better understanding of human pathology and neurodevelopmental disorders. This review describes the expression and function of Pax6 during brain development, and their implications for neuropathology.
Keywords: Pax6; Sey mutant; autism spectrum disorder; brain patterning; cell proliferation; central nervous system; mouse; neural differentiation; neural stem cells; rat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An allelic series at the paired box gene 6 (Pax6) locus reveals the functional specificity of Pax genes.J Biol Chem. 2013 Apr 26;288(17):12130-41. doi: 10.1074/jbc.M112.436865. Epub 2013 Mar 20. J Biol Chem. 2013. PMID: 23515312 Free PMC article.
-
Identification of pax6-dependent gene regulatory networks in the mouse lens.PLoS One. 2009;4(1):e4159. doi: 10.1371/journal.pone.0004159. Epub 2009 Jan 9. PLoS One. 2009. PMID: 19132093 Free PMC article.
-
Three new PAX6 mutations including one causing an unusual ophthalmic phenotype associated with neurodevelopmental abnormalities.Mol Vis. 2007 Apr 2;13:511-23. Mol Vis. 2007. PMID: 17417613 Free PMC article.
-
Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator.Stem Cells. 2008 Jul;26(7):1663-72. doi: 10.1634/stemcells.2007-0884. Epub 2008 May 8. Stem Cells. 2008. PMID: 18467663 Review.
-
Structural and functional consequences of PAX6 mutations in the brain: Implications for aniridia.Brain Res. 2021 Apr 1;1756:147283. doi: 10.1016/j.brainres.2021.147283. Epub 2021 Jan 28. Brain Res. 2021. PMID: 33515537 Review.
Cited by
-
Congenital aniridia beyond black eyes: From phenotype and novel genetic mechanisms to innovative therapeutic approaches.Prog Retin Eye Res. 2023 Jul;95:101133. doi: 10.1016/j.preteyeres.2022.101133. Epub 2022 Oct 22. Prog Retin Eye Res. 2023. PMID: 36280537 Free PMC article. Review.
-
SMC5 Plays Independent Roles in Congenital Heart Disease and Neurodevelopmental Disability.Int J Mol Sci. 2023 Dec 28;25(1):430. doi: 10.3390/ijms25010430. Int J Mol Sci. 2023. PMID: 38203602 Free PMC article.
-
Gene expression study in the siRNA based aniridia cell model and in primary aniridia limbal epithelial cells following duloxetine and ritanserin treatment.PLoS One. 2025 Jun 10;20(6):e0324829. doi: 10.1371/journal.pone.0324829. eCollection 2025. PLoS One. 2025. PMID: 40493651 Free PMC article.
-
Rare predicted deleterious FEZF2 variants are associated with a neurodevelopmental phenotype.Am J Med Genet A. 2024 Jul;194(7):e63578. doi: 10.1002/ajmg.a.63578. Epub 2024 Feb 29. Am J Med Genet A. 2024. PMID: 38425142 Free PMC article.
-
Identification of neural-relevant toxcast high-throughput assay intended gene targets: Applicability to neurotoxicity and neurotoxicant putative molecular initiating events.Neurotoxicology. 2024 Jul;103:256-265. doi: 10.1016/j.neuro.2024.07.001. Epub 2024 Jul 6. Neurotoxicology. 2024. PMID: 38977203 Free PMC article. Review.
References
-
- Ton C.C., Hirvonen H., Miwa H., Weil M.M., Monaghan P., Jordan T., van Heyningen V., Hastie N.D., Meijers-Heijboer H., Drechsler M., et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

