Maternal Prenatal Inflammation Increases Brain Damage Susceptibility of Lipopolysaccharide in Adult Rat Offspring via COX-2/PGD-2/DPs Pathway Activation
- PMID: 35682823
- PMCID: PMC9181626
- DOI: 10.3390/ijms23116142
Maternal Prenatal Inflammation Increases Brain Damage Susceptibility of Lipopolysaccharide in Adult Rat Offspring via COX-2/PGD-2/DPs Pathway Activation
Abstract
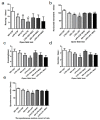
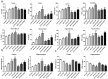
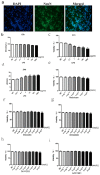
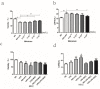
A growing body of research suggests that inflammatory insult contributes to the etiology of central nervous system diseases, such as depression, Alzheimer's disease, and so forth. However, the effect of prenatal systemic inflammation exposure on offspring brain development and cerebral susceptibility to inflammatory insult remains unknown. In this study, we utilized the prenatal inflammatory insult model in vivo and the neuronal damage model in vitro. The results obtained show that prenatal maternal inflammation exacerbates LPS-induced memory impairment, neuronal necrosis, brain inflammatory response, and significantly increases protein expressions of COX-2, DP2, APP, and Aβ, while obviously decreasing that of DP1 and the exploratory behaviors of offspring rats. Meloxicam significantly inhibited memory impairment, neuronal necrosis, oxidative stress, and inflammatory response, and down-regulated the expressions of APP, Aβ, COX-2, and DP2, whereas significantly increased exploring behaviors and the expression of DP1 in vivo. Collectively, these findings suggested that maternal inflammation could cause offspring suffering from inflammatory and behavioral disorders and increase the susceptibility of offspring to cerebral pathological factors, accompanied by COX-2/PGD-2/DPs pathway activation, which could be ameliorated significantly by COX-2 inhibitor meloxicam treatment.
Keywords: DPs; central nervous system inflammation; cyclooxygenase-2; lipopolysaccharide; prenatal maternal inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Sanchez P.E., Zhu L., Verret L., Vossel K.A., Orr A.G., Cirrito J.R., Devidze N., Ho K., Yu G.-Q., Palop J.J., et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. - DOI - PMC - PubMed
-
- Oprica M., Hjorth E., Spulber S., Popescu B.O., Ankarcrona M., Winblad B., Schultzberg M. Studies on brain volume, Alzheimer-related proteins and cytokines in mice with chronic overexpression of IL-1 receptor antagonist. J. Cell. Mol. Med. 2007;11:810–825. doi: 10.1111/j.1582-4934.2007.00074.x. - DOI - PMC - PubMed
-
- Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

