Butylidenephthalide Abrogates the Snail-Induced Cancer Stemness in Oral Carcinomas
- PMID: 35682836
- PMCID: PMC9180956
- DOI: 10.3390/ijms23116157
Butylidenephthalide Abrogates the Snail-Induced Cancer Stemness in Oral Carcinomas
Abstract
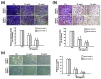
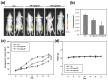
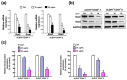
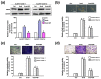
Oral cancer is one of the most common cancers worldwide, especially in South Central Asia. It has been suggested that cancer stem cells (CSC) play crucial roles in tumor relapse and metastasis, and approaches to target CSC may lead to promising results. Here, aldehyde dehydrogenase 1 (ALDH1) and CD44 were utilized to isolate CSCs of oral cancer. Butylidenephthalide, a bioactive phthalide compound from Angelica sinensis, was tested for its anti-CSC effects. MTT assay showed that a lower concentration of butylidenephthalide was sufficient to inhibit the proliferation of patient-derived ALDH1+/CD44+ cells without affecting normal cells. Administration of butylidenephthalide not only reduced ALDH1 activity and CD44 expression, it also suppressed the migration, invasion, and colony formation abilities of ALDH1+/CD44+ cells using a transwell system and clonogenic assay. A patient-derived xenograft mouse model supported our in vitro findings that butylidenephthalide possessed the capacity to retard tumor development. We found that butylidenephthalide dose-dependently downregulated the gene and protein expression of Sox2 and Snail. Our results demonstrated that overexpression of Snail in ALDH1-/CD44- (non-CSCs) cells induced the CSC phenotypes, whereas butylidenephthalide treatment successfully diminished the enhanced self-renewal and propagating properties. In summary, this study showed that butylidenephthalide may serve as an adjunctive for oral cancer therapy.
Keywords: ALDH1; CD44; Snail; butylidenephthalide; cancer stem cells; oral cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

