Social Isolation Stress Modulates Pregnancy Outcomes and the Inflammatory Profile of Rat Uterus
- PMID: 35682846
- PMCID: PMC9181517
- DOI: 10.3390/ijms23116169
Social Isolation Stress Modulates Pregnancy Outcomes and the Inflammatory Profile of Rat Uterus
Abstract
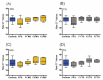
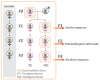
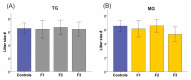
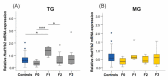
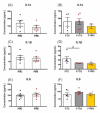
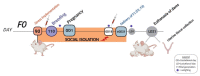
Prenatal stressors have been linked to adverse pregnancy outcomes; including preterm birth (PTB). Recent work demonstrates that social isolation in mothers represents a silent stressor contributing to PTB risk. Here; we investigate the association of inflammatory and stress markers with PTB risk in Long-Evans rats exposed to social isolation stress (SIS) during preconception and pregnancy across four generations (F0-F3). Gestational length; blood glucose; corticosterone levels; and maternal and offspring weights were assessed in two SIS paradigms: transgenerational (TG) and multigenerational (MG) exposure. Maternal uterine tissues were collected 21 days after the dams gave birth. Exposure to SIS reduced pregnancy lengths in the parental generation and neonatal birth weights in the F1 and F2 generations. Interleukin (IL)-1β (Il1b) mRNA levels increased in F0 animals but decreased in the offspring of both stress lineages. Protein levels of IL-1β decreased in the TG lineage. Corticotrophin-releasing hormone receptor 1 (Crhr1) expression decreased in SIS-exposed F0 animals and increased in the TG-F2 and MG-F1 offspring. Expression of enzyme 11-β hydroxysteroid dehydrogenase-2 (11bHSD2) was enhanced in F1 animals. These findings suggest SIS has adverse consequences on the F0 mothers; but their F1-F3 progeny may adapt to this chronic stress; thus supporting the fetal programming hypothesis.
Keywords: fetal programming; gene expression; inflammation; intrauterine growth restriction; pregnancy; prenatal stress; preterm birth; rodents; social isolation; uterus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

