Dicarbonyl Stress in Diabetic Vascular Disease
- PMID: 35682865
- PMCID: PMC9181283
- DOI: 10.3390/ijms23116186
Dicarbonyl Stress in Diabetic Vascular Disease
Abstract
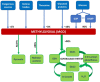
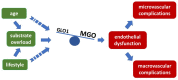
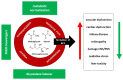
Late vascular complications play a prominent role in the diabetes-induced increase in morbidity and mortality. Diabetes mellitus is recognised as a risk factor driving atherosclerosis and cardiovascular mortality; even after the normalisation of blood glucose concentration, the event risk is amplified-an effect called "glycolytic memory". The hallmark of this glycolytic memory and diabetic pathology are advanced glycation end products (AGEs) and reactive glucose metabolites such as methylglyoxal (MGO), a highly reactive dicarbonyl compound derived mainly from glycolysis. MGO and AGEs have an impact on vascular and organ structure and function, contributing to organ damage. As MGO is not only associated with hyperglycaemia in diabetes but also with other risk factors for diabetic vascular complications such as obesity, dyslipidaemia and hypertension, MGO is identified as a major player in the development of vascular complications in diabetes both on micro- as well as macrovascular level. In diabetes mellitus, the detoxifying system for MGO, the glyoxalase system, is diminished, accounting for the increased MGO concentration and glycotoxic load. This overview will summarise current knowledge on the effect of MGO and AGEs on vascular function.
Keywords: advanced glycation end products; atherosclerotic disease; cardiovascular risk; endothelial dysfunction; glycotoxic load; methylglyoxal.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Tancredi M., Rosengren A., Svensson A.M., Kosiborod M., Pivodic A., Gudbjornsdottir S., Dahlqvist S., Wedel H., Lind M. Glycaemic control and excess mortality in patients with type 2 diabetes. Diabetologia. 2015;58:S137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

