The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19
- PMID: 35682871
- PMCID: PMC9181280
- DOI: 10.3390/ijms23116196
The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19
Abstract
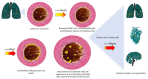
The endothelium has a fundamental role in the cardiovascular complications of coronavirus disease 2019 (COVID-19). Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) particularly affects endothelial cells. The virus binds to the angiotensin-converting enzyme 2 (ACE-2) receptor (present on type 2 alveolar cells, bronchial epithelial cells, and endothelial cells), and induces a cytokine storm. The cytokines tumor necrosis factor alpha, interleukin-1 beta, and interleukin-6 have particular effects on endothelial cells-leading to endothelial dysfunction, endothelial cell death, changes in tight junctions, and vascular hyperpermeability. Under normal conditions, apoptotic endothelial cells are removed into the bloodstream. During COVID-19, however, endothelial cells are detached more rapidly, and do not regenerate as effectively as usual. The loss of the endothelium on the luminal surface abolishes all of the vascular responses mediated by the endothelium and nitric oxide production in particular, which results in greater contractility. Moreover, circulating endothelial cells infected with SARS-CoV-2 act as vectors for viral dissemination by forming clusters that migrate into the circulation and reach distant organs. The cell clusters and the endothelial dysfunction might contribute to the various thromboembolic pathologies observed in COVID-19 by inducing the formation of intravascular microthrombi, as well as by triggering disseminated intravascular coagulation. Here, we review the contributions of endotheliopathy and endothelial-cell-derived extracellular vesicles to the pathogenesis of COVID-19, and discuss therapeutic strategies that target the endothelium in patients with COVID-19.
Keywords: COVID-19; SARS-CoV-2; endothelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

