Novel Epoxides of Soloxolone Methyl: An Effect of the Formation of Oxirane Ring and Stereoisomerism on Cytotoxic Profile, Anti-Metastatic and Anti-Inflammatory Activities In Vitro and In Vivo
- PMID: 35682893
- PMCID: PMC9181525
- DOI: 10.3390/ijms23116214
Novel Epoxides of Soloxolone Methyl: An Effect of the Formation of Oxirane Ring and Stereoisomerism on Cytotoxic Profile, Anti-Metastatic and Anti-Inflammatory Activities In Vitro and In Vivo
Abstract
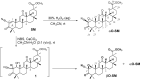
It is known that epoxide-bearing compounds display pronounced pharmacological activities, and the epoxidation of natural metabolites can be a promising strategy to improve their bioactivity. Here, we report the design, synthesis and evaluation of biological properties of αO-SM and βO-SM, novel epoxides of soloxolone methyl (SM), a cyanoenone-bearing derivative of 18βH-glycyrrhetinic acid. We demonstrated that the replacement of a double-bound within the cyanoenone pharmacophore group of SM with α- and β-epoxide moieties did not abrogate the high antitumor and anti-inflammatory potentials of the triterpenoid. It was found that novel SM epoxides induced the death of tumor cells at low micromolar concentrations (IC50(24h) = 0.7-4.1 µM) via the induction of mitochondrial-mediated apoptosis, reinforced intracellular accumulation of doxorubicin in B16 melanoma cells, probably by direct interaction with key drug efflux pumps (P-glycoprotein, MRP1, MXR1), and the suppressed pro-metastatic phenotype of B16 cells, effectively inhibiting their metastasis in a murine model. Moreover, αO-SM and βO-SM hampered macrophage functionality in vitro (motility, NO production) and significantly suppressed carrageenan-induced peritonitis in vivo. Furthermore, the effect of the stereoisomerism of SM epoxides on the mentioned bioactivities and toxic profiles of these compounds in vivo were evaluated. Considering the comparable antitumor and anti-inflammatory effects of SM epoxides with SM and reference drugs (dacarbazine, dexamethasone), αO-SM and βO-SM can be considered novel promising antitumor and anti-inflammatory drug candidates.
Keywords: 18βH-glycyrrhetinic acid; ABC transporters; CDDO-Me; anti-inflammatory activity; anti-metastatic activity; apoptosis; epoxide; pharmacophore group; soloxolone methyl; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Cyano Enone-Bearing Triterpenoid Soloxolone Methyl Inhibits Epithelial-Mesenchymal Transition of Human Lung Adenocarcinoma Cells In Vitro and Metastasis of Murine Melanoma In Vivo.Molecules. 2020 Dec 14;25(24):5925. doi: 10.3390/molecules25245925. Molecules. 2020. PMID: 33327637 Free PMC article.
-
Trioxolone Methyl, a Novel Cyano Enone-Bearing 18βH-Glycyrrhetinic Acid Derivative, Ameliorates Dextran Sulphate Sodium-Induced Colitis in Mice.Molecules. 2020 May 21;25(10):2406. doi: 10.3390/molecules25102406. Molecules. 2020. PMID: 32455822 Free PMC article.
-
Novel 3'-Substituted-1',2',4'-Oxadiazole Derivatives of 18βH-Glycyrrhetinic Acid and Their O-Acylated Amidoximes: Synthesis and Evaluation of Antitumor and Anti-Inflammatory Potential In Vitro and In Vivo.Int J Mol Sci. 2020 May 15;21(10):3511. doi: 10.3390/ijms21103511. Int J Mol Sci. 2020. PMID: 32429154 Free PMC article.
-
[Fatty acid epoxides in the regulation of the inflammation].Biomed Khim. 2022 Jun;68(3):177-189. doi: 10.18097/PBMC20226803177. Biomed Khim. 2022. PMID: 35717582 Review. Russian.
-
Metabolism and molecular toxicology of isoprene.Chem Biol Interact. 2001 Jun 1;135-136:223-38. doi: 10.1016/s0009-2797(01)00192-2. Chem Biol Interact. 2001. PMID: 11397393 Review.
Cited by
-
Complex Inhibitory Activity of Pentacyclic Triterpenoids against Cutaneous Melanoma In Vitro and In Vivo: A Literature Review and Reconstruction of Their Melanoma-Related Protein Interactome.ACS Pharmacol Transl Sci. 2024 Oct 23;7(11):3358-3384. doi: 10.1021/acsptsci.4c00422. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539268 Review.
-
Soloxolone Methyl Reduces the Stimulatory Effect of Leptin on the Aggressive Phenotype of Murine Neuro2a Neuroblastoma Cells via the MAPK/ERK1/2 Pathway.Pharmaceuticals (Basel). 2023 Sep 27;16(10):1369. doi: 10.3390/ph16101369. Pharmaceuticals (Basel). 2023. PMID: 37895840 Free PMC article.
-
Phytochemical studies of Gerbera jamesonii and evaluation of anti-inflammatory potential in formaldehyde-induced arthritis in rats.Inflammopharmacology. 2025 Jun;33(6):3213-3231. doi: 10.1007/s10787-025-01720-2. Epub 2025 Apr 3. Inflammopharmacology. 2025. PMID: 40178779
-
Soloxolone N-3-(Dimethylamino)propylamide Restores Drug Sensitivity of Tumor Cells with Multidrug-Resistant Phenotype via Inhibition of P-Glycoprotein Efflux Function.Molecules. 2024 Oct 18;29(20):4939. doi: 10.3390/molecules29204939. Molecules. 2024. PMID: 39459307 Free PMC article.
-
Soloxolone methyl induces apoptosis and oxidative/ER stress in breast cancer cells and target cancer stem cell population.Turk J Biol. 2023 Jun 5;47(4):247-261. doi: 10.55730/1300-0152.2660. eCollection 2023. Turk J Biol. 2023. PMID: 38152618 Free PMC article.
References
-
- Silverman R.B., Holladay M.W. The Organic Chemistry of Drug Design and Drug Action. Elsevier Science; Amsterdam, The Netherlands: 2014.
-
- Srivastava A., Maggs J.L., Antoine D.J., Williams D.P., Smith D.A., Park B.K. In: Role of Reactive Metabolites in Drug-Induced Hepatotoxicity BT—Adverse Drug Reactions. Uetrecht J., editor. Springer; Berlin/Heidelberg, Germany: 2010. pp. 165–194. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

