Venetoclax Induces Cardiotoxicity through Modulation of Oxidative-Stress-Mediated Cardiac Inflammation and Apoptosis via NF-κB and BCL-2 Pathway
- PMID: 35682939
- PMCID: PMC9181135
- DOI: 10.3390/ijms23116260
Venetoclax Induces Cardiotoxicity through Modulation of Oxidative-Stress-Mediated Cardiac Inflammation and Apoptosis via NF-κB and BCL-2 Pathway
Abstract
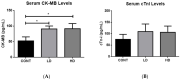
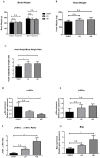
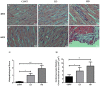
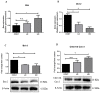
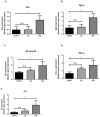
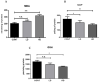
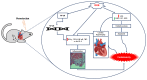
Cardiovascular damage induced by anticancer therapy has become the main health problem after tumor elimination. Venetoclax (VTX) is a promising novel agent that has been proven to have a high efficacy in multiple hematological diseases, especially acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL). Considering its mechanism of action, the possibility that VTX may cause cardiotoxicity cannot be ruled out. Therefore, this study was designed to investigate the toxic effect of VTX on the heart. Male Sprague-Dawley rats were randomly divided into three groups: control, low-dose VTX (50 mg/kg via oral gavage), and high-dose VTX (100 mg/kg via oral gavage). After 21 days, blood and tissue samples were collected for histopathological, biochemical, gene, and protein analyses. We demonstrated that VTX treatment resulted in cardiac damages as evidenced by major changes in histopathology and markedly elevated cardiac enzymes and hypertrophic genes markers. Moreover, we observed a drastic increase in oxidative stress, as well as inflammatory and apoptotic markers, with a remarkable decline in the levels of Bcl-2. To the best of our knowledge, this study is the first to report the cardiotoxic effect of VTX. Further experiments and future studies are strongly needed to comprehensively understand the cardiotoxic effect of VTX.
Keywords: apoptosis; cardiotoxicity; inflammation; oxidative stress; venetoclax.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Laverty H.G., Benson C., Cartwright E.J., Cross M.J., Garland C., Hammond T., Holloway C., McMahon N., Milligan J., Park B.K., et al. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br. J. Pharmacol. 2011;163:675–693. doi: 10.1111/j.1476-5381.2011.01255.x. - DOI - PMC - PubMed
-
- Kim H., Chung W.B., Im Cho K., Kim B.J., Seo J.S., Park S.M., Kim H.J., Lee J.H., Kim E.K., Youn H.J. Diagnosis, Treatment, and Prevention of Cardiovascular Toxicity Related to Anti-Cancer Treatment in Clinical Practice: An Opinion Paper from the Working Group on Cardio-Oncology of the Korean Society of Echocardiography. J. Cardiovasc. Ultrasound. 2018;26:1–25. doi: 10.4250/jcu.2018.26.1.1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

