Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin-Thiadiazole Hybrids
- PMID: 35682998
- PMCID: PMC9180949
- DOI: 10.3390/ijms23116314
Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin-Thiadiazole Hybrids
Abstract
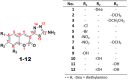
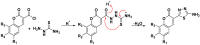
A novel series of coumarin-thiadiazole hybrids, derived from substituted coumarin-3-carboxylic acids was isolated and fully characterized with the use of a number of spectroscopic techniques and XRD crystallography. Several of the novel compounds showed intensive fluorescence in the visible region, comparable to that of known coumarin-based fluorescence standards. Moreover, the new compounds were tested as potential antineurodegenerative agents via their ability to act as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitors. Compared to the commercial standards, only a few compounds demonstrated moderate AChE and BuChE activities. Moreover, the novel derivatives were tested for their antimicrobial activity against a panel of pathogenic bacterial and fungal species. Their lack of activity and toxicity across a broad range of biochemical assays, together with the exceptional emission of some hybrid molecules, highlights the possible use of a number of the novel hybrids as potential fluorescence standards or fluorescence imaging agents.
Keywords: antimicrobial activity; cholinesterase inhibitors; coumarin; fluorescence imaging; hybrids; neurodegeneration; thiadiazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
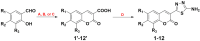




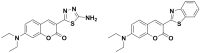
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

