Benzo[ a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity
- PMID: 35683027
- PMCID: PMC9181839
- DOI: 10.3390/ijms23116348
Benzo[ a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity
Abstract
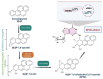
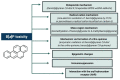
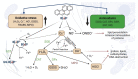
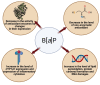
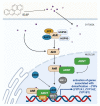
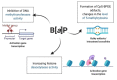
Benzo[a]pyrene (B[a]P) is the main representative of polycyclic aromatic hydrocarbons (PAHs), and has been repeatedly found in the air, surface water, soil, and sediments. It is present in cigarette smoke as well as in food products, especially when smoked and grilled. Human exposure to B[a]P is therefore common. Research shows growing evidence concerning toxic effects induced by this substance. This xenobiotic is metabolized by cytochrome P450 (CYP P450) to carcinogenic metabolite: 7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE), which creates DNA adducts, causing mutations and malignant transformations. Moreover, B[a]P is epigenotoxic, neurotoxic, and teratogenic, and exhibits pro-oxidative potential and causes impairment of animals' fertility. CYP P450 is strongly involved in B[a]P metabolism, and it is simultaneously expressed as a result of the association of B[a]P with aromatic hydrocarbon receptor (AhR), playing an essential role in the cancerogenic potential of various xenobiotics. In turn, polymorphism of CYP P450 genes determines the sensitivity of the organism to B[a]P. It was also observed that B[a]P facilitates the multiplication of viruses, which may be an additional problem with the widespread COVID-19 pandemic. Based on publications mainly from 2017 to 2022, this paper presents the occurrence of B[a]P in various environmental compartments and human surroundings, shows the exposure of humans to this substance, and describes the mechanisms of its toxicity.
Keywords: benzo[a]pyrene; carcinogenicity; genotoxicity; metabolism; polycyclic aromatic hydrocarbons.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Bezza F.A., Chirwa E.M.N. The Role of Lipopeptide Biosurfactant on Microbial Remediation of Aged Polycyclic Aromatic Hydrocarbons (PAHs)-Contaminated Soil. Chem. Eng. J. 2017;309:563–576. doi: 10.1016/j.cej.2016.10.055. - DOI
-
- Saravanakumar K., Sivasantosh S., Sathiyaseelan A., Sankaranarayanan A., Naveen K.V., Zhang X., Jamla M., Vijayasarathy S., Vishnu Priya V., MubarakAli D., et al. Impact of Benzo[a]Pyrene with Other Pollutants Induce the Molecular Alternation in the Biological System: Existence, Detection and Remediation Methods. Environ. Pollut. 2022;304:119207. doi: 10.1016/j.envpol.2022.119207. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

