Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik-Fields Reaction
- PMID: 35683150
- PMCID: PMC9182137
- DOI: 10.3390/ma15113846
Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik-Fields Reaction
Abstract
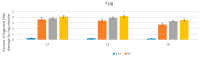
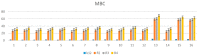
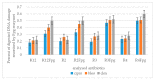
We reported a new method dealing with the synthesis of novel pharmacologically relevant α-aminophosphonate derivatives via a lipase-catalyzed Kabachnik−Fields reaction with yields of up to 93%. The advantages of this protocol are excellent yields, mild reaction conditions, low costs, and sustainability. The developed protocol is applicable to a range of H-phosphites and organic amines, providing a wide substrate scope. A new class of α-aminophosphonate analogues possessing P-chiral centers was also synthesized. The synthesized compounds were characterized on the basis of their antimicrobial activities against E. coli. The impact of the various alkoxy groups on antimicrobial activity was demonstrated. The crucial role of the substituents, located at the aromatic rings in the phenylethyloxy and benzyloxy groups, on the inhibitory action against selected pathogenic E. coli strains was revealed. The observed results are especially important because of increasing resistance of bacteria to various drugs and antibiotics.
Keywords: Kabachnik−Fields reaction; antimicrobial activity; α-aminophosphonates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
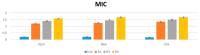


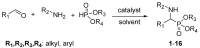



References
-
- Hiratake J., Oda J. Aminophosphonic and Aminoboronic Acids as Key Elements of a Transition State Analogue Inhibitor of Enzymes. Biosci. Biotechnol. Biochem. 1997;61:211–218. doi: 10.1271/bbb.61.211. - DOI
-
- Kafarski P., Lejczak B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991;63:193–215. doi: 10.1080/10426509108029443. - DOI
-
- Nassan M.A., Aldhahrani A., Amer H.H., Elhenawy A., Swelum A.A., Ali O.M., Zaki Y.H. Investigation of the Anticancer Effect of α-Aminophosphonates and Arylidine Derivatives of 3-Acetyl-1-aminoquinolin-2(1H)-one on the DMBA Model of Breast Cancer in Albino Rats with In Silico Prediction of Their Thymidylate Synthase Inhibitory Effect. Molecules. 2022;27:756. doi: 10.3390/molecules27030756. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

