Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS
- PMID: 35683340
- PMCID: PMC9180983
- DOI: 10.3390/jcm11112951
Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS
Abstract
Background: Glucocorticoids (GCs) have been shown to reduce mortality and the need for invasive mechanical ventilation (IMV) in SARS-CoV-2-induced acute respiratory distress syndrome (ARDS). It has been suggested that serum cytokines levels are markers of disease severity in ARDS, although there is only limited evidence of a relationship between the longitudinal cytokine profile and clinical outcomes in patients with SARS-CoV-2-induced ARDS treated with GC.
Methods: We conducted a single-center observational study to investigate serial plasma cytokine levels in 17 patients supported with non-invasive ventilation (NIV) in order to compare the response in five patients who progressed to IMV versus 12 patients who continued with NIV alone. All patients received methylprednisolone 80 mg/day continuous infusion until clinical improvement.
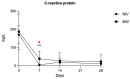
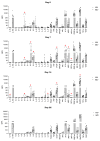
Results: The study groups were comparable at baseline. All patients survived. Although IL-6 was higher in the NIV group at baseline, several cytokines were significantly higher in the IMV group on day 7 (IL-6, IL-8, IL-9, G-CSF, IP-10, MCP-1, MIP-1α) and 14 (IL-6, IL-8, IL-17, G-CSF, MIP-1α, RANTES). No significant differences were observed between groups on day 28.
Conclusions: Patients in the IMV group had higher inflammation levels at intubation than the NIV group, which may indicate a higher resistance to glucocorticoids. Higher GC doses or a longer treatment duration in these patients might have allowed for a better control of inflammation and a better outcome. Further studies are required to define the prognostic value of cytokine patterns, in terms of both GC treatment tailoring and timely initiation of IMV.
Keywords: COVID-19; acute respiratory distress syndrome (ARDS); cytokines; glucocorticoids; non-invasive ventilation (NIV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pelosi P., Tonelli R., Torregiani C., Baratella E., Confalonieri M., Battaglini D., Marchioni A., Confalonieri P., Clini E., Salton F., et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022;11:1704. doi: 10.3390/jcm11061704. - DOI - PMC - PubMed
-
- Ioannou G.N., Locke E., Green P., Berry K., O’Hare A.M., Shah J.A., Crothers K., Eastment M.C., Dominitz J.A., Fan V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death among 10131 US Veterans with SARS-CoV-2 Infection. JAMA Netw. Open. 2020;3:2022310. doi: 10.1001/jamanetworkopen.2020.22310. - DOI - PMC - PubMed
-
- Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O., et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. - DOI - PMC - PubMed
-
- RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., et al. Dexamethasone in Hospitalized Patients with COVID-19—Preliminary Report. N. Engl. J. Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

