Synthesis of a Two-Dimensional Molybdenum Disulfide Nanosheet and Ultrasensitive Trapping of Staphylococcus Aureus for Enhanced Photothermal and Antibacterial Wound-Healing Therapy
- PMID: 35683721
- PMCID: PMC9182539
- DOI: 10.3390/nano12111865
Synthesis of a Two-Dimensional Molybdenum Disulfide Nanosheet and Ultrasensitive Trapping of Staphylococcus Aureus for Enhanced Photothermal and Antibacterial Wound-Healing Therapy
Abstract
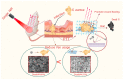
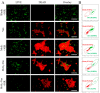
Photothermal therapy has been widely used in the treatment of bacterial infections. However, the short photothermal effective radius of conventional nano-photothermal agents makes it difficult to achieve effective photothermal antibacterial activity. Therefore, improving composite targeting can significantly inhibit bacterial growth. We inhibited the growth of Staphylococcus aureus (S. aureus) by using an extremely low concentration of vancomycin (Van) and applied photothermal therapy with molybdenum disulfide (MoS2). This simple method used chitosan (CS) to synthesize fluorescein 5(6)-isothiocyanate (FITC)-labeled and Van-loaded MoS2-nanosheet hydrogels (MoS2-Van-FITC@CS). After modifying the surface, an extremely low concentration of Van could inhibit bacterial growth by trapping bacteria synergistically with the photothermal effects of MoS2, while FITC labeled bacteria and chitosan hydrogels promoted wound healing. The results showed that MoS2-Van-FITC@CS nanosheets had a thickness of approximately 30 nm, indicating the successful synthesis of the nanosheets. The vitro antibacterial results showed that MoS2-Van-FITC with near-infrared irradiation significantly inhibited S. aureus growth, reaching an inhibition rate of 94.5% at nanoparticle concentrations of up to 100 µg/mL. Furthermore, MoS2-Van-FITC@CS could exert a healing effect on wounds in mice. Our results demonstrate that MoS2-Van-FITC@CS is biocompatible and can be used as a wound-healing agent.
Keywords: MoS2 nanosheet; antibacterial activity; photothermal therapy; wound-healing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Photothermal antibacterial MoS2 composited chitosan hydrogel for infectious wound healing.Biomater Adv. 2024 Jan;156:213701. doi: 10.1016/j.bioadv.2023.213701. Epub 2023 Nov 20. Biomater Adv. 2024. PMID: 38039808
-
An NIR-II-enhanced nanozyme to promote wound healing in methicillin-resistant Staphylococcus aureus infections.Acta Biomater. 2024 Apr 15;179:300-312. doi: 10.1016/j.actbio.2024.03.014. Epub 2024 Mar 20. Acta Biomater. 2024. PMID: 38518865
-
Engineering Injectable Coassembled Hydrogel by Photothermal Driven Chitosan-Stabilized MoS2 Nanosheets for Infected Wound Healing.ACS Nano. 2024 Oct 1;18(39):26961-26974. doi: 10.1021/acsnano.4c08883. Epub 2024 Sep 21. ACS Nano. 2024. PMID: 39305262
-
Recent advances in MoS2-based photothermal therapy for cancer and infectious disease treatment.J Mater Chem B. 2020 Jul 15;8(27):5793-5807. doi: 10.1039/d0tb01018a. J Mater Chem B. 2020. PMID: 32597915 Review.
-
MoS2 based nanomaterials: Advanced antibacterial agents for future.J Control Release. 2022 Aug;348:158-185. doi: 10.1016/j.jconrel.2022.05.047. Epub 2022 Jun 7. J Control Release. 2022. PMID: 35662576 Review.
Cited by
-
Luminescence Nanomaterials and Applications.Nanomaterials (Basel). 2023 Mar 14;13(6):1047. doi: 10.3390/nano13061047. Nanomaterials (Basel). 2023. PMID: 36985939 Free PMC article.
-
Recent Progress of Photothermal Therapy Based on Conjugated Nanomaterials in Combating Microbial Infections.Nanomaterials (Basel). 2023 Aug 7;13(15):2269. doi: 10.3390/nano13152269. Nanomaterials (Basel). 2023. PMID: 37570588 Free PMC article. Review.
-
Recent advances in reactive oxygen species scavenging nanomaterials for wound healing.Exploration (Beijing). 2024 Jan 17;4(3):20230066. doi: 10.1002/EXP.20230066. eCollection 2024 Jun. Exploration (Beijing). 2024. PMID: 38939866 Free PMC article. Review.
-
Two-dimensional nanomaterials: A multifunctional approach for robust for diabetic wound repair.Mater Today Bio. 2024 Aug 6;28:101186. doi: 10.1016/j.mtbio.2024.101186. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39221220 Free PMC article. Review.
-
Advancements in employing two-dimensional nanomaterials for enhancing skin wound healing: a review of current practice.J Nanobiotechnology. 2024 Aug 30;22(1):520. doi: 10.1186/s12951-024-02803-y. J Nanobiotechnology. 2024. PMID: 39210430 Free PMC article. Review.
References
-
- Li W., Song P., Xin Y., Kuang Z., Liu Q., Ge F., Zhu L., Zhang X., Tao Y., Zhang W. The Effects of Luminescent CdSe Quantum Dot-Functionalized Antimicrobial Peptides Nanoparticles on Antibacterial Activity and Molecular Mechanism. Int. J. Nanomed. 2021;16:1849–1867. doi: 10.2147/IJN.S295928. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

