Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes
- PMID: 35683856
- PMCID: PMC9182857
- DOI: 10.3390/polym14112183
Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes
Abstract
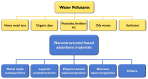
Chemical water pollution poses a threat to human beings and ecological systems. The purification of water to remove toxic organic and inorganic pollutants is essential for a safe society and a clean environment. Adsorption-based water treatment is considered one of the most effective and economic technologies designed to remove toxic substances. In this article, we review the recent progress in the field of nanostructured materials used for water purification, particularly those used for the adsorption of heavy metal ions and organic dyes. This review includes a range of nanostructured materials such as metal-based nanoparticles, polymer-based nanomaterials, carbon nanomaterials, bio-mass materials, and other types of nanostructured materials. Finally, the current challenges in the fields of adsorption of toxic materials using nanostructured materials are briefly discussed.
Keywords: heavy metal adsorption; nanostructured materials; organic dye adsorption; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Mitigation of environmentally-related hazardous pollutants from water matrices using nanostructured materials - A review.Chemosphere. 2020 Aug;253:126770. doi: 10.1016/j.chemosphere.2020.126770. Epub 2020 Apr 12. Chemosphere. 2020. PMID: 32464768 Review.
-
Recent Advances of Metal-Organic Framework for Heavy Metal Ions Adsorption.Langmuir. 2024 Aug 13. doi: 10.1021/acs.langmuir.4c01757. Online ahead of print. Langmuir. 2024. PMID: 39136076 Review.
-
Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review.Environ Res. 2018 Apr;162:173-195. doi: 10.1016/j.envres.2017.12.025. Epub 2018 Jan 9. Environ Res. 2018. PMID: 29329014 Review.
-
A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously.Bioresour Technol. 2017 Dec;245(Pt A):649-655. doi: 10.1016/j.biortech.2017.09.016. Epub 2017 Sep 5. Bioresour Technol. 2017. PMID: 28917099
-
Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal-organic frameworks: A review of studies from the last decade.Chemosphere. 2018 Jun;201:627-643. doi: 10.1016/j.chemosphere.2018.03.047. Epub 2018 Mar 6. Chemosphere. 2018. PMID: 29544217 Review.
Cited by
-
Methylene Blue and Rhodamine B Dyes' Efficient Removal Using Biocarbons Developed from Waste.Molecules. 2024 Aug 25;29(17):4022. doi: 10.3390/molecules29174022. Molecules. 2024. PMID: 39274870 Free PMC article.
-
Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review.Materials (Basel). 2022 Aug 5;15(15):5392. doi: 10.3390/ma15155392. Materials (Basel). 2022. PMID: 35955327 Free PMC article. Review.
-
Review on the impact of heavy metals from industrial wastewater effluent and removal technologies.Heliyon. 2024 Nov 15;10(23):e40370. doi: 10.1016/j.heliyon.2024.e40370. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654720 Free PMC article. Review.
-
Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation.Polymers (Basel). 2022 Oct 1;14(19):4121. doi: 10.3390/polym14194121. Polymers (Basel). 2022. PMID: 36236070 Free PMC article.
-
Approaches for the Efficient Removal of Fluoride from Groundwater: A Comprehensive Review.Toxics. 2024 Apr 23;12(5):306. doi: 10.3390/toxics12050306. Toxics. 2024. PMID: 38787085 Free PMC article. Review.
References
-
- Gupta V.K., Ali I., Saleh T.A., Nayak A., Agarwal S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012;2:6380–6388. doi: 10.1039/c2ra20340e. - DOI
-
- Ranade V.V., Bhandari V.M. Industrial Wastewater Treatment, Recycling and Reuse. Butterworth-Heinemann; Oxford, UK: 2014.
-
- [(accessed on 14 February 2022)]. Available online: https://public.wmo.int/en/media/press-release/wake-looming-water-crisis-....
-
- ISO . ISO/TS 80004-1: Nanotechnologies—Vocabulary—Part 1: Core Terms. ISO (the International Organization for Standardization); Geneva, Switzerland: 2015.
Publication types
LinkOut - more resources
Full Text Sources

