A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA)
- PMID: 35683928
- PMCID: PMC9182759
- DOI: 10.3390/polym14112254
A Theoretical Study on the Phosgenation of 2,4-Toluenediamine (2,4-TDA)
Abstract
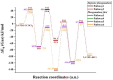
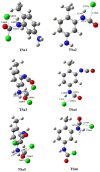
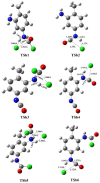
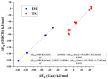
Industrially relevant phosgenation mechanisms of 2,4-toluenediamine (2,4-TDA) were investigated using G3MP2B3 model chemistry. Six reaction pathways had been explored, which resulted in the formation of toluene diisocyanate (2,4-TDI) including different scenarios of the 'phosgenations first' and 'consecutive phosgenations' mechanisms in both gas and condensed phases. Two possible 'phosgenations first' mechanisms show superior to the others in terms of energy, regardless of which phases are considered. Due to the o-dichlorobenzene (ODCB) solvation, the reaction barriers are dramatically reduced compared to the gas-phase reaction mechanism and the solvent effect can be described by linear relationship. Standard enthalpy of formation value was also recommended for 2,4-TDA (59.3 kJ/mol) and 2,4-TDI (-94.1 kJ/mol), as well as for the gas-phase intermediates (IM).
Keywords: G3MP2B3; carbamic chloride; phosgenation; toluene diisocyanate; toluenediamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
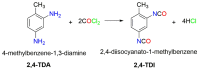




References
-
- Golling F.E., Pires R., Hecking A., Weikard J., Richter F., Danielmeier K., Dijkstra D. Polyurethanes for Coatings and Adhesives—Chemistry and Applications. Polym. Int. 2019;68:848–855. doi: 10.1002/pi.5665. - DOI
-
- Parod R.J. Toluene Diisocyanate. 3rd ed. Volume 4. Elsevier; Amsterdam, The Netherlands: 2014.
-
- TDI Global Demand 2021|Statista. [(accessed on 26 April 2022)]. Available online: https://www.statista.com/statistics/750818/tdi-demand-worldwide/
-
- Lorenz W., Padeken L., Pennemann B., Steffens F., Weismantel L. Process for the Preparation of Toluene-Diisocyanate. 8063241B2. U.S. Patent. 2011 November 22;
Grants and funding
LinkOut - more resources
Full Text Sources

