Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities
- PMID: 35683965
- PMCID: PMC9182795
- DOI: 10.3390/polym14112293
Synthesis of Chitosan-Based Gold Nanoparticles: Antimicrobial and Wound-Healing Activities
Abstract
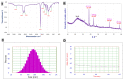
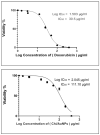
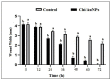
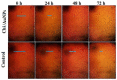
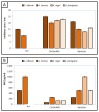
The global spread of multidrug-resistant bacteria has become a significant hazard to public health, and more effective antibacterial agents are required. Therefore, this study describes the preparation, characterization, and evaluation of gold nanoparticles modified with chitosan (Chi/AuNPs) as a reducing and stabilizing agent with efficient antimicrobial effects. In recent years, the development of an efficient and ecofriendly method for synthesizing metal nanoparticles has attracted a lot of interest in the field of nanotechnology. Colloidal gold nanoparticles (AuNPs) were prepared by the chemical reduction of gold ions in the presence of chitosan (Chi), giving Chi/AuNPs. The characterization of Chi/AuNPs was carried out by transmission electron microscopy (TEM), scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), and X-ray diffraction (XRD). Chi/AuNPs appeared spherical and monodispersed, with a diameter ranging between 20 to 120 nm. The synergistic effects of AuNPs and Chi led to the disruption of bacterial membranes. The maximum inhibitory impact was seen against P. aeruginosa at 500 µg/mL, with a zone of inhibition diameter of 26 ± 1.8 mm, whereas the least inhibitory effect was reported for S. aureus, with a zone of inhibition diameter of 16 ± 2.1 mm at the highest dose tested. Moreover, Chi/AuNPs exhibited antifungal activity toward Candida albicans when the MIC was 62.5 µg/mL. Cell viability and proliferation of the developed nanocomposite were evaluated using a sulphorhodamine B (SRB) assay with a half inhibitory concentration (IC50) of 111.1 µg/mL. Moreover, the in vitro wound-healing model revealed that the Chi/AuNP dressing provides a relatively rapid and efficacious wound-healing ability, making the obtained nanocomposite a promising candidate for the development of improved bandage materials.
Keywords: antibacterial activity; antifungal activity; chitosan; cytotoxicity; nanocomposite; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jim O. Tackling a Crisis for the Future Health and Wealth of Nations. Review on Antimicrobial Resistance; London, UK: 2014.
-
- Puebla L.E.J. Immunodeficiency. IntechOpen; London, UK: 2012. Fungal infections in immunosuppressed patients.
LinkOut - more resources
Full Text Sources

