Graphene Oxide/Polyvinyl Alcohol-Formaldehyde Composite Loaded by Pb Ions: Structure and Electrochemical Performance
- PMID: 35683975
- PMCID: PMC9183114
- DOI: 10.3390/polym14112303
Graphene Oxide/Polyvinyl Alcohol-Formaldehyde Composite Loaded by Pb Ions: Structure and Electrochemical Performance
Abstract
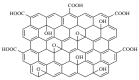
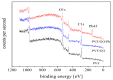
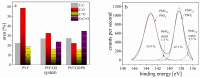
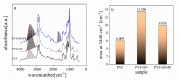
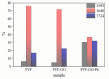
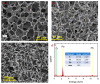
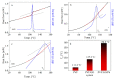
An immobilization of graphene oxide (GO) into a matrix of polyvinyl formaldehyde (PVF) foam as an eco-friendly, low cost, superior, and easily recovered sorbent of Pb ions from an aqueous solution is described. The relationships between the structure and electrochemical properties of PVF/GO composite with implanted Pb ions are discussed for the first time. The number of alcohol groups decreased by 41% and 63% for PVF/GO and the PVF/GO/Pb composite, respectively, compared to pure PVF. This means that chemical bonds are formed between the Pb ions and the PVF/GO composite based on the OH groups. This bond formation causes an increase in the Tg values attributed to the formation of a strong surface complexation between adjacent layers of PVF/GO composite. The conductivity increases by about 2.8 orders of magnitude compared to the values of the PVF/GO/Pb composite compared to the PVF. This means the presence of Pb ions is the main factor for enhancing the conductivity where the conduction mechanism is changed from ionic for PVF to electronic conduction for PVF/GO and PVF/GO/Pb.
Keywords: conductivity; dynamic mobility; graphene oxide; lead ions; polyvinyl formaldehyde.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
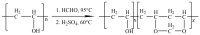


References
-
- Abdel-Baset T., Hekal E., Azab A., Anis B. Broadband dielectric properties of polyvinyl-formaldehyde/MWCNTs foams. Phys. B Condens. Matter. 2020;604:412666. doi: 10.1016/j.physb.2020.412666. - DOI
-
- Arbatti M., Shan X., Cheng Z.-Y. Ceramic–Polymer Composites with High Dielectric Constant. Adv. Mater. 2007;19:1369–1372. doi: 10.1002/adma.200601996. - DOI
-
- Long B., Balogun M.-S., Luo L., Qiu W., Luo Y., Song S., Tong Y. Phase Boundary Derived Pseudocapacitance Enhanced Nickel-Based Composites for Electrochemical Energy Storage Devices. Adv. Energy Mater. 2017;8 doi: 10.1002/aenm.201701681. - DOI
-
- Zhao B., Hamidinejad M., Zhao C., Li R., Wang S., Kazemi Y., Park C.B. A versatile foaming platform to fabricate polymer/carbon composites with high dielectric permittivity and ultra-low dielectric loss. J. Mater. Chem. A. 2018;7:133–140. doi: 10.1039/C8TA05556D. - DOI
-
- Zhou L., Fu Q., Xue F., Tang X., Zhou D., Tian Y., Wang G., Wang C., Gou H., Xu L. Multiple Interfacial Fe3O4@BaTiO3/P(VDF-HFP) Core–Shell–Matrix Films with Internal Barrier Layer Capacitor (IBLC) Effects and High Energy Storage Density. ACS Appl. Mater. Interfaces. 2017;9:40792–40800. doi: 10.1021/acsami.7b10923. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

