Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study
- PMID: 35684003
- PMCID: PMC9182401
- DOI: 10.3390/nu14112203
Fermented Carica papaya and Morinda citrifolia as Perspective Food Supplements for the Treatment of Post-COVID Symptoms: Randomized Placebo-Controlled Clinical Laboratory Study
Abstract
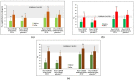
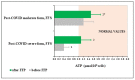
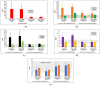
Food supplements based on fermented Carica papaya and Morinda citrifolia, known for their immune modulating, redox balancing, and anti-inflammatory effects, were added to conventional treatment protocols prescribed to patients recovering after severe and moderate COVID-19 disease in order to alleviate long-lasting post-COVID symptoms. A randomized single-center placebo-controlled clinical laboratory study was designed and performed (total number of participants 188, with delta variant of virus 157, with omicron 31). Clinical statuses were assessed using computer tomography, electrocardiography, a questionnaire, and physical endurance. Plasma cytokines (IL-6, IL-8, IL-17A, and INF-gamma), nitrate/nitrite ratio, antioxidant activity (AOA), and polymorphonuclear leukocyte (PMN) ATP levels were determined before and 20 days following the addition of 28 g of fermented supplements twice per day. The capacity of PMN to phagocyte and the oral-nasal-pharyngeal microbiota were assessed. Clinical symptoms, IL-6, IL-8, and nitric oxide metabolites diminished significantly compared to the placebo group and their background expression. The PMN capacity to phagocyte, AOA, and ATP content remarkably increased. The oral-nasal-pharyngeal microbiota were unchanged. On these grounds, we suggest that fermented tropical fruits could efficiently diminish post-COVID clinical symptoms through several immune-modulating, redox balancing, and pro-energy mechanisms.
Keywords: ATP; Carica papaya; Morinda citrifolia; antioxidant activity; fermented food supplements; nitrates/nitrites; oral-nasal-pharyngeal microbiota; phagocytosis; post-COVID symptoms; pro-inflammatory cytokines.
Conflict of interest statement
J.C. is a senior researcher of Natural Health Farm, Selangor, Malaysia, which partly funded this clinical laboratory study. The funder had no role in the conceptualisation of the study and its design, in the collection, analyses, and interpretation of the data, in the writing of the manuscript, and in the decision to publish the results. The authors declare no conflict of interest.
Figures


Similar articles
-
Comparative activity of antioxidants from wheat sprouts, Morinda citrifolia, fermented papaya and white tea.Int J Food Sci Nutr. 2006 May-Jun;57(3-4):168-77. doi: 10.1080/09637480600658328. Int J Food Sci Nutr. 2006. PMID: 17127467
-
A purely green synthesis of silver nanoparticles using Carica papaya, Manihot esculenta, and Morinda citrifolia: synthesis and antibacterial evaluations.Bioprocess Biosyst Eng. 2017 Sep;40(9):1349-1361. doi: 10.1007/s00449-017-1793-z. Epub 2017 Jun 8. Bioprocess Biosyst Eng. 2017. PMID: 28597212
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Nutraceutical Potential of Carica papaya in Metabolic Syndrome.Nutrients. 2019 Jul 16;11(7):1608. doi: 10.3390/nu11071608. Nutrients. 2019. PMID: 31315213 Free PMC article. Review.
-
Applications and bioefficacy of the functional food supplement fermented papaya preparation.Toxicology. 2010 Nov 28;278(1):6-16. doi: 10.1016/j.tox.2010.09.006. Epub 2010 Sep 24. Toxicology. 2010. PMID: 20870007 Review.
Cited by
-
The effects of supplementation of noni (Morinda citrifolia L.) fruit polysaccharides-rich extract on antioxidant status and immune function in cashmere goats.J Anim Sci. 2022 Oct 1;100(10):skac276. doi: 10.1093/jas/skac276. J Anim Sci. 2022. PMID: 35998071 Free PMC article.
-
A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery.Nutrients. 2025 May 26;17(11):1802. doi: 10.3390/nu17111802. Nutrients. 2025. PMID: 40507071 Free PMC article.
-
Interventions for Long COVID: A Narrative Review.J Gen Intern Med. 2025 Jul;40(9):2005-2023. doi: 10.1007/s11606-024-09254-z. Epub 2025 Feb 21. J Gen Intern Med. 2025. PMID: 39984803 Review.
-
The Impact of Previous Comorbidities on New Comorbidities and Medications after a Mild SARS-CoV-2 Infection in a Lithuanian Cohort.J Clin Med. 2024 Jan 22;13(2):623. doi: 10.3390/jcm13020623. J Clin Med. 2024. PMID: 38276129 Free PMC article.
-
Dietary Polysaccharide-Rich Extract from Noni (Morinda citrifolia L.) Fruit Modified Ruminal Fermentation, Ruminal Bacterial Community and Nutrient Digestion in Cashmere Goats.Animals (Basel). 2023 Jan 6;13(2):221. doi: 10.3390/ani13020221. Animals (Basel). 2023. PMID: 36670760 Free PMC article.
References
-
- Fodor A., Tiperciuc B., Login C., Orasan O.H., Lazar A.L., Buchman C., Hanghicel P., Sitar-Taut A., Suharoschi R., Vulturar R., et al. Endothelial dysfunction, inflammation, and oxidative stress in COVID-19-mechanisms and therapeutic targets. Oxid. Med. Cell. Longev. 2021;2021:8671713. doi: 10.1155/2021/8671713. - DOI - PMC - PubMed
-
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

