Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming
- PMID: 35684292
- PMCID: PMC9182836
- DOI: 10.3390/plants11111520
Respiratory and Photosynthetic Responses of Antarctic Vascular Plants Are Differentially Affected by CO2 Enrichment and Nocturnal Warming
Abstract
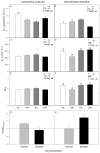
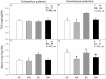
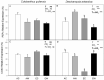
Projected rises in atmospheric CO2 concentration and minimum night-time temperatures may have important effects on plant carbon metabolism altering the carbon balance of the only two vascular plant species in the Antarctic Peninsula. We assessed the effect of nocturnal warming (8/5 °C vs. 8/8 °C day/night) and CO2 concentrations (400 ppm and 750 ppm) on gas exchange, non-structural carbohydrates, two respiratory-related enzymes, and mitochondrial size and number in two species of vascular plants. In Colobanthus quitensis, light-saturated photosynthesis measured at 400 ppm was reduced when plants were grown in the elevated CO2 or in the nocturnal warming treatments. Growth in elevated CO2 reduced stomatal conductance but nocturnal warming did not. The short-term sensitivity of respiration, relative protein abundance, and mitochondrial traits were not responsive to either treatment in this species. Moreover, some acclimation to nocturnal warming at ambient CO2 was observed. Altogether, these responses in C. quitensis led to an increase in the respiration-assimilation ratio in plants grown in elevated CO2. The response of Deschampsia antarctica to the experimental treatments was quite distinct. Photosynthesis was not affected by either treatment; however, respiration acclimated to temperature in the elevated CO2 treatment. The observed short-term changes in thermal sensitivity indicate type I acclimation of respiration. Growth in elevated CO2 and nocturnal warming resulted in a reduction in mitochondrial numbers and an increase in mitochondrial size in D. antarctica. Overall, our results suggest that with climate change D. antarctica could be more successful than C. quitensis, due to its ability to make metabolic adjustments to maintain its carbon balance.
Keywords: Antarctic plant species; atmospheric CO2 concentration; foliar carbon balance; nocturnal warming; photosynthesis; respiration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ciais P., Sabine C., Bala G., Bopp L., Brovkin V., Canadell J., Chhabra A., DeFries R., Galloaway J., Heimann M., et al. Carbon and other biogeochemical cycles. In: Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernamental Panel on Climate Change. Cambridge University Press; Cambridge, UK: New York, NY, USA: 2013. pp. 465–570.
-
- IPCC . Summary for Policymakers. In: Masson-Delmotte V., Zhai P., Pörtner H.O., Roberts D., Skea J., Shukla P.R., Pirani A., Moufouma-Okia W., Péan C., Pidcock R.S., et al., editors. Global Warming of 1.5 °C. IPCC; Geneva, Switzerland: 2018.
-
- Bracegirdle T.J., Barrand N.E., Kusahara K., Wainer I. Predicting Antarctic climate using climate models. [(accessed on 6 September 2020)];Antarct. Environ. Portal. 2016 Available online: http://nora.nerc.ac.uk/id/eprint/513739.
-
- IPCC . Technical Summary. In: Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K.B., Tignor M., Miller H.L., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: New York, NY, USA: 2017.
Grants and funding
LinkOut - more resources
Full Text Sources

