Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering
- PMID: 35684380
- PMCID: PMC9182049
- DOI: 10.3390/molecules27113442
Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering
Abstract
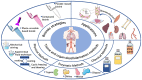
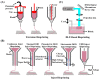
Three-dimensional (3D) bioprinting is one of the most promising additive manufacturing technologies for fabricating various biomimetic architectures of tissues and organs. In this context, the bioink, a critical element for biofabrication, is a mixture of biomaterials and living cells used in 3D printing to create cell-laden structures. Recently, decellularized extracellular matrix (dECM)-based bioinks derived from natural tissues have garnered enormous attention from researchers due to their unique and complex biochemical properties. This review initially presents the details of the natural ECM and its role in cell growth and metabolism. Further, we briefly emphasize the commonly used decellularization treatment procedures and subsequent evaluations for the quality control of the dECM. In addition, we summarize some of the common bioink preparation strategies, the 3D bioprinting approaches, and the applicability of 3D-printed dECM bioinks to tissue engineering. Finally, we present some of the challenges in this field and the prospects for future development.
Keywords: 3D bioprinting; bioink; decellularized extracellular matrix; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Yang D., Lu B., Zhao Y., Jiang X. Fabrication of aligned fibirous arrays by magnetic electrospinning. Adv. Mater. 2007;19:3702. doi: 10.1002/adma.200700171. - DOI
-
- Yamada M., Utoh R., Ohashi K., Tatsumi K., Yamato M., Okano T., Seki M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials. 2012;33:8304–8315. doi: 10.1016/j.biomaterials.2012.07.068. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

