Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress
- PMID: 35684511
- PMCID: PMC9182196
- DOI: 10.3390/molecules27113576
Synthesis of Cyclic Fragrances via Transformations of Alkenes, Alkynes and Enynes: Strategies and Recent Progress
Abstract
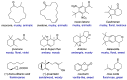
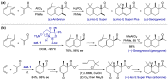
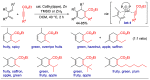
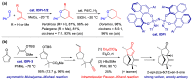
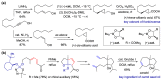
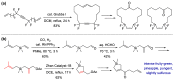
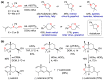
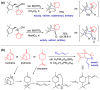
With increasing demand for customized commodities and the greater insight and understanding of olfaction, the synthesis of fragrances with diverse structures and odor characters has become a core task. Recent progress in organic synthesis and catalysis enables the rapid construction of carbocycles and heterocycles from readily available unsaturated molecular building blocks, with increased selectivity, atom economy, sustainability and product diversity. In this review, synthetic methods for creating cyclic fragrances, including both natural and synthetic ones, will be discussed, with a focus on the key transformations of alkenes, alkynes, dienes and enynes. Several strategies will be discussed, including cycloaddition, catalytic cyclization, ring-closing metathesis, intramolecular addition, and rearrangement reactions. Representative examples and the featured olfactory investigations will be highlighted, along with some perspectives on future developments in this area.
Keywords: asymmetric organocatalysis; carbocycles; cascade/tandem/domino reaction; cyclization; cycloaddition; heterocycles; natural fragrances; synthetic odorants; transition-metal catalysis; unsaturated hydrocarbons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









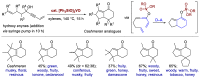
Similar articles
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
-
Bioconjugation with strained alkenes and alkynes.Acc Chem Res. 2011 Sep 20;44(9):805-15. doi: 10.1021/ar200059z. Epub 2011 Jul 18. Acc Chem Res. 2011. PMID: 21766804
-
Tandem sequence of cross metathesis--ring-closing metathesis reaction of alkynyl silyloxy-tethered enynes.J Am Chem Soc. 2005 Jul 6;127(26):9410-5. doi: 10.1021/ja0520159. J Am Chem Soc. 2005. PMID: 15984868
-
Asymmetric synthesis of densely functionalized medium-ring carbocycles and lactones through modular assembly and ring-closing metathesis of sulfoximine-substituted trienes and dienynes.Chemistry. 2012 Mar 19;18(12):3529-48. doi: 10.1002/chem.201103060. Epub 2012 Feb 16. Chemistry. 2012. PMID: 22345000
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
Cited by
-
Continuous Flow Epoxidation of Alkenes Using a Homogeneous Manganese Catalyst with Peracetic Acid.Org Process Res Dev. 2023 Jan 14;27(2):262-268. doi: 10.1021/acs.oprd.2c00222. eCollection 2023 Feb 17. Org Process Res Dev. 2023. PMID: 36844035 Free PMC article. Review.
-
Do Synthetic Fragrances in Personal Care and Household Products Impact Indoor Air Quality and Pose Health Risks?J Xenobiot. 2023 Mar 1;13(1):121-131. doi: 10.3390/jox13010010. J Xenobiot. 2023. PMID: 36976159 Free PMC article. Review.
-
Engineering carboxylic acid reductases and unspecific peroxygenases for flavor and fragrance biosynthesis.J Biotechnol. 2024 Apr 10;385:1-12. doi: 10.1016/j.jbiotec.2024.02.013. Epub 2024 Feb 28. J Biotechnol. 2024. PMID: 38428504 Free PMC article. Review.
-
Thiadiazole chitosan conjugates as a novel cosmetic ingredient for rinse-off hair conditioners: design, formulation, characterization and in silico-molecular docking studies.BMC Chem. 2025 Apr 19;19(1):104. doi: 10.1186/s13065-025-01404-6. BMC Chem. 2025. PMID: 40253399 Free PMC article.
-
Hydrogenation of cyclohexene over single-atom Pt or Pd incorporated porous ceria nanoparticles under solvent-free conditions.RSC Adv. 2024 Apr 2;14(15):10644-10652. doi: 10.1039/d4ra01432d. eCollection 2024 Mar 26. RSC Adv. 2024. PMID: 38567333 Free PMC article.
References
-
- Schilling B., Kaiser R., Natsch A., Gautschi M. Investigation of Odors in the Fragrance Industry. Chemoecology. 2010;20:135–147. doi: 10.1007/s00049-009-0035-5. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

