Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications
- PMID: 35684512
- PMCID: PMC9182285
- DOI: 10.3390/molecules27113574
Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications
Abstract
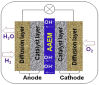
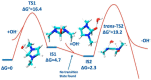
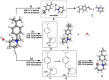
Anion Exchange Membrane (AEM) fuel cells have attracted growing interest, due to their encouraging advantages, including high power density and relatively low cost. AEM is a polymer matrix, which conducts hydroxide (OH-) ions, prevents physical contact of electrodes, and has positively charged head groups (mainly quaternary ammonium (QA) groups), covalently bound to the polymer backbone. The chemical instability of the quaternary ammonium (QA)-based head groups, at alkaline pH and elevated temperature, is a significant threshold in AEMFC technology. This review work aims to introduce recent studies on the chemical stability of various QA-based head groups and transportation of OH- ions in AEMFC, via modeling and simulation techniques, at different scales. It starts by introducing the fundamental theories behind AEM-based fuel-cell technology. In the main body of this review, we present selected computational studies that deal with the effects of various parameters on AEMs, via a variety of multi-length and multi-time-scale modeling and simulation methods. Such methods include electronic structure calculations via the quantum Density Functional Theory (DFT), ab initio, classical all-atom Molecular Dynamics (MD) simulations, and coarse-grained MD simulations. The explored processing and structural parameters include temperature, hydration levels, several QA-based head groups, various types of QA-based head groups and backbones, etc. Nowadays, many methods and software packages for molecular and materials modeling are available. Applications of such methods may help to understand the transportation mechanisms of OH- ions, the chemical stability of functional head groups, and many other relevant properties, leading to a performance-based molecular and structure design as well as, ultimately, improved AEM-based fuel cell performances. This contribution aims to introduce those molecular modeling methods and their recent applications to the AEM-based fuel cells research community.
Keywords: anion exchange membrane; chemical stability; fuel cell; modeling; multi-scale; transportation mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
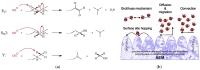




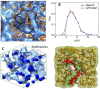
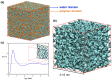
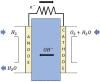
References
-
- Kober T., Schiffer H.W., Densing M., Panos E. Global energy perspectives to 2060–WEC’s World Energy Scenarios 2019. Energy Strategy Rev. 2020;31:100523. doi: 10.1016/j.esr.2020.100523. - DOI
-
- Dutta S. A review on production, storage of hydrogen and its utilization as an energy resource. Ind. Eng. Chem. Res. 2014;20:1148–1156. doi: 10.1016/j.jiec.2013.07.037. - DOI
-
- Spiegel C. Designing and Building Fuel Cells. Volume 87 McGraw-Hill Professional; New York, NY, USA: 2007.
-
- Breeze P. Fuel Cells. 1st ed. Academic Press; Cambridge, MA, USA: 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

