Synthesis and Anticancer Properties of New 3-Methylidene-1-sulfonyl-2,3-dihydroquinolin-4(1 H)-ones
- PMID: 35684532
- PMCID: PMC9181899
- DOI: 10.3390/molecules27113597
Synthesis and Anticancer Properties of New 3-Methylidene-1-sulfonyl-2,3-dihydroquinolin-4(1 H)-ones
Abstract
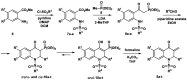
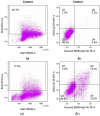
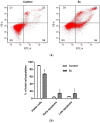
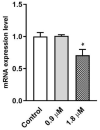
Quinolinones have been known for a long time as broad-spectrum synthetic antibiotics. More recently, the anticancer potential of this group of compounds has been investigated. Following this direction, we obtained a small library of 3-methylidene-1-sulfonyl-2,3-dihydroquinolin-4(1H)-ones with various substituents at positions 1, 2, 6, and 7 of the quinolinone ring system. The cytotoxic activity of the synthesized analogs was tested in the MTT assay on two cancer cell lines in order to determine the structure-activity relationship. All compounds produced high cytotoxic effects in MCF-7, and even higher in HL-60 cells. 2-Ethyl-3-methylidene-1-phenylsulfonyl-2,3-dihydroquinolin-4(1H)-one, which was over 5-fold more cytotoxic for HL-60 than for normal HUVEC cells, was selected for further tests. This analog was shown to inhibit proliferation and induce DNA damage and apoptosis in HL-60 cells.
Keywords: Horner–Wadsworth–Emmons olefination; apoptosis; cytotoxicity; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
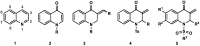


References
-
- Kahriman N., Iskender N.Y., Yucel M., Yayli N., Demir E., Demirbag Z. Microwave-assisted synthesis of 1,3′-diaza-flavanone/flavone and their alkyl derivatives with antimicrobial activity. J. Heterocycl. Chem. 2012;49:71–79. doi: 10.1002/jhet.800. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

