The Use of Antibiotics as Chiral Selectors in Capillary Electrophoresis: A Review
- PMID: 35684535
- PMCID: PMC9181903
- DOI: 10.3390/molecules27113601
The Use of Antibiotics as Chiral Selectors in Capillary Electrophoresis: A Review
Abstract
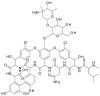
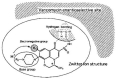
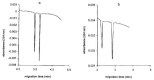
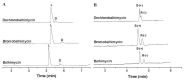
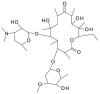
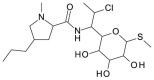
Chirality is becoming an essential issue in modern pharmaceutical research as regulatory agencies emphasize the safety and efficiency of enantiomers in drug development. The development of efficient and reliable chiral separation methods became a necessity in the last 30 years, and capillary electrophoresis (CE), due to its relatively low costs and "green" features, is attracting increased attention. Cyclodextrin (CD) and their derivatives are the most frequently used chiral selectors (CSs) in CE, however, the use of antibiotics as CSs represents an interesting alternative. Various classes of antibiotics (aminoglycosides, ansamycins, glycopeptides, lincosamides, macrolides, tetracyclines) have been used more or less successfully for the enantio-separation of pharmaceuticals. Antibiotics offer the possibility of a multitude of potential interactions (electrostatic, inclusion, hydrogen bonding, etc.) due to their chemical diversity, allowing the enantio-separation of analytes with a wide range of structural characteristics. This article aims to review the application of various classes of antibiotics in the CE enantio-separation of pharmaceuticals. Antibiotic physiochemical characteristics, variables impacting enantio-separation, advantages, and disadvantages when certain antibiotics are used as CSs in CE are also explored.
Keywords: antibiotics; capillary electrophoresis; chiral drugs; chiral selectors; chiral separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sekhon B.S. Exploiting the power of stereochemistry in drugs: An overview of racemic and enantiopure drugs. J. Mod. Med. Chem. 2013;1:10–36. doi: 10.12970/2308-8044.2013.01.01.2. - DOI
-
- U.S. Food and Drug Administration: Guidance document Development of New Stereoisomeric Drugs, 1992. [(accessed on 1 February 2022)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
-
- Núñez M.D.C., Gallo M.A., Espinosa A., Campos J.M. Rapid Development of Chiral Drugs in the Pharmaceutical Industry. New Developments in Medicinal Chemistry. Bentham Science Publishers; Sharjah, United Arab Emirates: 2010. pp. 95–113.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

