An Electrochemical Sensor Based on Carbon Paper Modified with Graphite Powder for Sensitive Determination of Sunset Yellow and Tartrazine in Drinks
- PMID: 35684711
- PMCID: PMC9185310
- DOI: 10.3390/s22114092
An Electrochemical Sensor Based on Carbon Paper Modified with Graphite Powder for Sensitive Determination of Sunset Yellow and Tartrazine in Drinks
Abstract
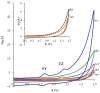
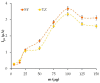
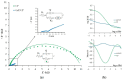
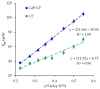
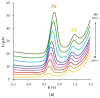
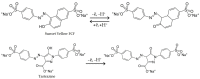
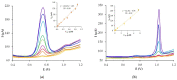
The paper describes the development of an electrochemical sensor to be used for the determination of synthetic food colorants such as Sunset Yellow FCF (SY) and Tartrazine (TZ). The sensor is a carbon paper (CP) electrode, manufactured by using hot lamination technology and volume modified with fine-grained graphite powder (GrP). The sensor (GrP/CP) was characterized by scanning electron microscopy, energy dispersive spectrometry, electrochemical impedance analysis, cyclic, linear sweep and differential pulse voltammetry. The mechanism of SY and TZ electrochemical oxidation on GrP/CP was studied. The developed sensor has good electron transfer characteristics and low electron resistance, high sensitivity and selectivity. Applying the differential pulse mode, linear dynamic ranges of 0.005-1.0 μM and 0.02-7.5 μM with limits of detection of 0.78 nM and 8.2 nM for SY and TZ, respectively, were obtained. The sensor was used to detect SY and TZ in non-alcoholic and alcoholic drinks. The results obtained from drink analysis prove good reproducibility (RSD ≤ 0.072) and accuracy (recovery 96-104%).
Keywords: Sunset Yellow; Tartrazine; carbon paper; carbon veil; electrochemical sensor; food colorants; graphite powder; modified electrode; soft and alcoholic drinks; voltammetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages.Talanta. 2016 Nov 1;160:113-124. doi: 10.1016/j.talanta.2016.07.011. Epub 2016 Jul 5. Talanta. 2016. PMID: 27591594
-
Fabrication of β-cyclodextrin-coated poly (diallyldimethylammonium chloride)-functionalized graphene composite film modified glassy carbon-rotating disk electrode and its application for simultaneous electrochemical determination colorants of sunset yellow and tartrazine.Anal Chim Acta. 2013 May 24;779:22-34. doi: 10.1016/j.aca.2013.03.061. Epub 2013 Apr 6. Anal Chim Acta. 2013. PMID: 23663668
-
Simultaneous determination of Sunset yellow and Tartrazine in soft drinks using gold nanoparticles carbon paste electrode.Food Chem. 2012 May 1;132(1):637-41. doi: 10.1016/j.foodchem.2011.10.103. Epub 2011 Nov 7. Food Chem. 2012. PMID: 26434343
-
Latest advances on the nanomaterials-based electrochemical analysis of azo toxic dyes Sunset Yellow and Tartrazine in food samples.Food Chem Toxicol. 2021 Oct;156:112524. doi: 10.1016/j.fct.2021.112524. Epub 2021 Aug 26. Food Chem Toxicol. 2021. PMID: 34454997 Review.
-
A comprehensive exploration of tartrazine detection in food products: Leveraging fluorescence nanomaterials and electrochemical sensors: Recent progress and future trends.Food Chem. 2024 Feb 1;433:137425. doi: 10.1016/j.foodchem.2023.137425. Epub 2023 Sep 9. Food Chem. 2024. PMID: 37690141 Review.
Cited by
-
Analytical Applicability of Graphene-Modified Electrode in Sunset Yellow Electrochemical Assay.Sensors (Basel). 2023 Feb 14;23(4):2160. doi: 10.3390/s23042160. Sensors (Basel). 2023. PMID: 36850755 Free PMC article.
-
Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF.Sensors (Basel). 2024 Jan 31;24(3):930. doi: 10.3390/s24030930. Sensors (Basel). 2024. PMID: 38339646 Free PMC article.
-
Aqueous Two-Phase Systems Based on Cationic and Anionic Surfactants Mixture for Rapid Extraction and Colorimetric Determination of Synthetic Food Dyes.Sensors (Basel). 2023 Mar 28;23(7):3519. doi: 10.3390/s23073519. Sensors (Basel). 2023. PMID: 37050583 Free PMC article.
-
Recent advances in electrochemical detection of common azo dyes.Forensic Toxicol. 2025 Jan;43(1):1-21. doi: 10.1007/s11419-024-00696-y. Epub 2024 Aug 2. Forensic Toxicol. 2025. PMID: 39093537 Review.
-
Ponceau 4R elimination from fruit juice: An integrated optimization strategy utilizing artificial neural networks, least squares, and chitosan-nickel ferrite Nano Sorbent.Food Chem X. 2024 Sep 25;24:101856. doi: 10.1016/j.fochx.2024.101856. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39416305 Free PMC article.
References
-
- Martins N., Roriz C.L., Morales P., Barros L., Ferreira I.C. Food colorants: Challenges, opportunities and current desires of agroindustries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016;52:1–15. doi: 10.1016/j.tifs.2016.03.009. - DOI
-
- Rovina K., Acung L.A., Siddiquee S., Akanda J.H., Shaarani S.M. Extraction and analytical methods for determination of Sunset Yellow (E110)—A review. Food Anal. Methods. 2017;10:773–787. doi: 10.1007/s12161-016-0645-9. - DOI
-
- Okafor S.N., Obonga W., Ezeokonkwo M.A., Nurudeen J., Orovwigho U., Ahiabuike J. Assessment of the health implications of synthetic and natural food colourants—A critical review. Pharm. Biosci. J. 2016;4:1–11. doi: 10.20510/ukjpb/4/i4/110639. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

