Lycorine Inhibits Hypertrophic Scar Formation by Inducing ROS-Mediated Apoptosis
- PMID: 35685086
- PMCID: PMC9171077
- DOI: 10.3389/fbioe.2022.892015
Lycorine Inhibits Hypertrophic Scar Formation by Inducing ROS-Mediated Apoptosis
Abstract
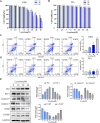
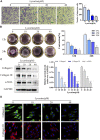
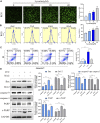
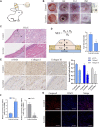
Background: Hypertrophic scar (HS) is a fibrotic cutaneous disease with few effective therapies. Lycorine is a drug with pro-apoptotic ability and anti-fibrosis potential. This study aimed to test whether lycorine could trigger the apoptosis of hypertrophic scar fibroblasts (HSFs) to inhibit HS formation. Methods: The proapoptotic and anti-fibrosis effects of lycorine on the viability and apoptosis of human primary HSFs and their reactive oxygen species (ROS) production as well as a rabbit ear model of HS were determined by CCK-8, flow cytometry, Western blot, immunofluorescence, transwell migration, collagen gel contraction assays. Results: Lycorine treatment selectively decreased the viability of HSFs, and induced their apoptosis, but not normal fibroblasts (NFs). Lycorine treatment increased the relative levels of Bax and cleaved PARP expression, cytochrome C cytoplasm translocation, but decreased Bcl-2, caspase-3 and caspase-9 expression, the mitochondrial membrane potential (MMP) in HSFs. Lycorine inhibited the migration and contraction of HSFs, and reduced the expression of collagen I, collagen III and α-SMA. Mechanistically, lycorine treatment stimulated high levels of ROS production, leading to apoptosis of HSFs while treatment with NAC, a ROS inhibitor, significantly mitigated or abrogated the pro-apoptotic and antifibrotic activity of lycorine in HSFs. Moreover, lycorine treatment mitigated the severity of HS in rabbit ears by inducing fibroblast apoptosis. Conclusion: These results indicate that lycorine has a potent anti-fibrotic activity and is a potential drug for intervention of HS.
Keywords: apoptosis; fibrosis; hypertrophic scar; lycorine; reactive oxygen species.
Copyright © 2022 Dong, Lv, Zhao, Xu, Hu and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

