Bisphenol S Alters the Steroidome in the Preovulatory Follicle, Oviduct Fluid and Plasma in Ewes With Contrasted Metabolic Status
- PMID: 35685208
- PMCID: PMC9172638
- DOI: 10.3389/fendo.2022.892213
Bisphenol S Alters the Steroidome in the Preovulatory Follicle, Oviduct Fluid and Plasma in Ewes With Contrasted Metabolic Status
Abstract
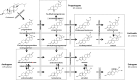
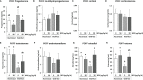
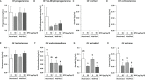
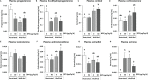
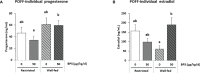
Bisphenol A (BPA), a plasticizer and endocrine disruptor, has been substituted by bisphenol S (BPS), a structural analogue that had already shown adverse effects on granulosa cell steroidogenesis. The objective of this study was to assess the effect of chronic exposure to BPS, a possible endocrine disruptor, on steroid hormones in the ovary, oviduct and plasma using the ewe as a model. Given the interaction between steroidogenesis and the metabolic status, the BPS effect was tested according to two diet groups. Eighty adult ewes were allotted to restricted (R) and well-fed (WF) groups, that were further subdivided into two subgroups. Ewes were exposed to 50 µg BPS/kg/day in their diet (R50 and WF50 groups) or were unexposed controls (R0 and WF0 groups). After at least 3 months of BPS exposure, preovulatory follicular fluid, oviduct fluid and plasma were collected and steroid hormones were analyzed by gas chromatography coupled with tandem mass spectrometry (GC-MS/MS). A deleterious effect of restricted diet on the volume of oviduct fluid and numbers of pre-ovulatory follicles was observed. Exposure to BPS impaired estradiol concentrations in both follicular and oviduct fluids of well-fed ewes and progesterone, estradiol and estrone concentrations in plasma of restricted ewes. In addition, a significant interaction between metabolic status and BPS exposure was observed for seven steroids, including estradiol. In conclusion, BPS acts in ewes as an endocrine disruptor with differential actions according to metabolic status.
Keywords: bisphenol S; endocrine disruptors; ewe; metabolic status; steroidome.
Copyright © 2022 Téteau, Liere, Pianos, Desmarchais, Lasserre, Papillier, Vignault, Lebachelier de la Riviere, Maillard, Binet, Uzbekova, Saint-Dizier and Elis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

