Facile synthesis of amides via acceptorless dehydrogenative coupling of aryl epoxides and amines
- PMID: 35685791
- PMCID: PMC9132053
- DOI: 10.1039/d2sc01959k
Facile synthesis of amides via acceptorless dehydrogenative coupling of aryl epoxides and amines
Abstract
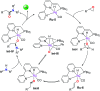
The synthesis of amides is significant in a wide variety of academic and industrial fields. We report here a new reaction, namely acceptorless dehydrogenative coupling of epoxides and amines to form amides catalyzed by ruthenium pincer complexes. Various aryl epoxides and amines smoothly convert into the desired amides in high yields with the generation of H2 gas as the only byproduct. Control experiments indicate that amides are generated kinetically faster than side products, possibly because of the facile activation of epoxides by metal-ligand cooperation, as supported by the observation of a ruthenium-enolate species. No alcohol or free aldehyde are involved. A mechanism is proposed involving a dual role of the catalyst, which is responsible for the high yield and selectivity of the new reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Material Science, ed. A. Greenberg, C. M. Breneman and J. F. Liebman, Wiley, New York, 2000
- Humphrey J. M. Chamberlin A. R. Chem. Rev. 1997;97:2243–2266. doi: 10.1021/cr950005s. - DOI - PubMed
- Bray B. L. Nat. Rev. Drug Discovery. 2003;2:587–593. doi: 10.1038/nrd1133. - DOI - PubMed
- Clader J. W. J. Med. Chem. 2004;47:1–9. doi: 10.1021/jm030283g. - DOI - PubMed
- Dunetz J. R. Magano J. Weisenburger G. A. Org. Process Res. Dev. 2016;20:140–177. doi: 10.1021/op500305s. - DOI
-
-
For reviews, see:
- Han S.-Y. Kim Y.-A. Tetrahedron. 2004;60:2447–2467. doi: 10.1016/j.tet.2004.01.020. - DOI
- Montalbetti C. A. G. N. Falque V. Tetrahedron. 2005;61:10827–10852. doi: 10.1016/j.tet.2005.08.031. - DOI
- Valeur E. Bradley M. Chem. Soc. Rev. 2009;38:606–631. doi: 10.1039/B701677H. - DOI - PubMed
- Lanigan R. M. Sheppard T. D. Eur. J. Org. Chem. 2013;33:7453–7465. doi: 10.1002/ejoc.201300573. - DOI
-
-
-
For reviews, see:
- Allen C. L. Williams J. M. J. Chem. Soc. Rev. 2011;40:3405–3415. doi: 10.1039/C0CS00196A. - DOI - PubMed
- Pattabiraman V. R. Bode J. W. Nature. 2011;480:471–479. doi: 10.1038/nature10702. - DOI - PubMed
- Huang L. Arndt M. Goobβn K. Heydt H. Goobβn L. J. Chem. Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u. - DOI - PubMed
- de Figueiredo R. M. Suppo J.-S. Campagne J.-M. Chem. Rev. 2016;116:12029–12122. doi: 10.1021/acs.chemrev.6b00237. - DOI - PubMed
-
-
-
For selective examples of catalytic synthesis of amides, see:
- Yoo W.-J. Li C.-J. J. Am. Chem. Soc. 2006;128:13064–13065. doi: 10.1021/ja064315b. - DOI - PubMed
- Gunanathan C. Ben-David Y. Milstein D. Science. 2007;317:790–792. doi: 10.1126/science.1145295. - DOI - PubMed
- Nakao Y. Idei H. Kanyiva K. S. Hiyama T. J. Am. Chem. Soc. 2009;131:5070–5071. doi: 10.1021/ja901153s. - DOI - PubMed
- Stephenson N. A. Zhu J. Gellman S. H. Stahl S. S. J. Am. Chem. Soc. 2009;131:10003–10008. doi: 10.1021/ja8094262. - DOI - PubMed
- Shen B. Makley D. M. Johnston J. N. Nature. 2010;465:1027–1032. doi: 10.1038/nature09125. - DOI - PMC - PubMed
- Gnanaprakasam B. Milstein D. J. Am. Chem. Soc. 2011;133:1682–1685. doi: 10.1021/ja109944n. - DOI - PubMed
- Fang X. Jackstell R. Beller M. Angew. Chem., Int. Ed. 2013;52:14089–14093. doi: 10.1002/anie.201308455. - DOI - PubMed
- Khusnutdinova J. R. Ben-David Y. Milstein D. J. Am. Chem. Soc. 2014;136:2998–3001. doi: 10.1021/ja500026m. - DOI - PubMed
-
-
- Nielsen L. P. C., Jacobsen E. N., Crotti P., Pineschi M., Olofsson B. and Somfai P., Aziridines and Epoxides in Organic Synthesis, ed. A. K. Yudin, Wiley-VCH, Weinheim, Germany, 2006, pp. 229–343
- Huang C.-Y. Doyle A. G. Chem. Rev. 2014;114:8153–8198. doi: 10.1021/cr500036t. - DOI - PubMed
- Jat J. L. Kumar G. Adv. Synth. Catal. 2019;361:4426–4441. doi: 10.1002/adsc.201900392. - DOI
- Hubbell A. K. Coates G. W. J. Org. Chem. 2020;85:13391–13414. doi: 10.1021/acs.joc.0c01691. - DOI - PubMed

