Impact of Extensively Hydrolyzed Infant Formula on Circulating Lipids During Early Life
- PMID: 35685890
- PMCID: PMC9171511
- DOI: 10.3389/fnut.2022.859627
Impact of Extensively Hydrolyzed Infant Formula on Circulating Lipids During Early Life
Abstract
Background: Current evidence suggests that the composition of infant formula (IF) affects the gut microbiome, intestinal function, and immune responses during infancy. However, the impact of IF on circulating lipid profiles in infants is still poorly understood. The objectives of this study were to (1) investigate how extensively hydrolyzed IF impacts serum lipidome compared to conventional formula and (2) to associate changes in circulatory lipids with gastrointestinal biomarkers including intestinal permeability.
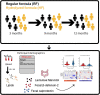
Methods: In a randomized, double-blind controlled nutritional intervention study (n = 73), we applied mass spectrometry-based lipidomics to analyze serum lipids in infants who were fed extensively hydrolyzed formula (HF) or conventional, regular formula (RF). Serum samples were collected at 3, 9, and 12 months of age. Child's growth (weight and length) and intestinal functional markers, including lactulose mannitol (LM) ratio, fecal calprotectin, and fecal beta-defensin, were also measured at given time points. At 3 months of age, stool samples were analyzed by shotgun metagenomics.
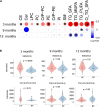
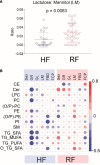
Results: Concentrations of sphingomyelins were higher in the HF group as compared to the RF group. Triacylglycerols (TGs) containing saturated and monounsaturated fatty acyl chains were found in higher levels in the HF group at 3 months, but downregulated at 9 and 12 months of age. LM ratio was lower in the HF group at 9 months of age. In the RF group, the LM ratio was positively associated with ether-linked lipids. Such an association was, however, not observed in the HF group.
Conclusion: Our study suggests that HF intervention changes the circulating lipidome, including those lipids previously found to be associated with progression to islet autoimmunity or overt T1D.
Clinical trial registration: [Clinicaltrials.gov], identifier [NCT01735123].
Keywords: early life; extensively hydrolyzed infant formula; intestinal permeability; lipidome; lipidomics; metabolomics.
Copyright © 2022 Lamichhane, Siljander, Salonen, Ruohtula, Virtanen, Ilonen, Hyötyläinen, Knip and Orešič.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. (2012) 2012:Cd003517. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

