Coronavirus Disease 2019 Vaccine Booster Effects Are Seen in Human Milk Antibody Response
- PMID: 35685893
- PMCID: PMC9171392
- DOI: 10.3389/fnut.2022.898849
Coronavirus Disease 2019 Vaccine Booster Effects Are Seen in Human Milk Antibody Response
Abstract
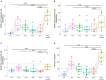
Infants remain at high risk for severe coronavirus disease 2019 (COVID-19). Human milk contains high levels of protective SARS CoV-2 specific antibodies post-infection and primary vaccine series, but levels decline over time. We hypothesized that the COVID-19 booster vaccine augment antibody production and the protection afforded to human milk-fed infants. We prospectively enrolled pregnant or lactating mothers planning to receive COVID-19 vaccination. We measured human milk IgG, IgA, and IgM antibodies targeting the SARS CoV-2 receptor binding domain within the spike protein and human milk neutralization activity against SARS CoV-2 in 10 lactating mothers from pre-COVID-19 primary series vaccine to post-booster dose. Human milk SARS CoV-2 specific IgG increased significantly from pre- to post-booster levels (median OD 0.33 vs. 2.02, P = 0.002). The IgG levels post-booster were even higher than the peak level after the primary series (2.02 vs. 0.95, P = 0.03). The increase in SARS CoV-2 specific IgA levels was not significant (0.10 vs. 0.33, P = 0.23). There was a strong correlation between paired maternal blood and milk IgG and IgA levels (IgG rho 0.52, P < 0.001, IgA rho 0.31, P = 0.05). Post-booster neutralizing activity was elevated compared to pre-booster levels (66% vs. 12% inhibition, P = 0.002). COVID-19 vaccine booster elicits SARS CoV-2 specific antibodies in human milk at higher levels compared to the initial primary series. This finding suggests that three doses of COVID-19 mRNA vaccination leads to improved mucosal response in human milk and reinforces current guidance recommending all pregnant or lactating mothers receive full COVID-19 vaccine courses with a booster dose.
Keywords: COVID-19; IgA; breastfeeding; breastmilk; immunization; infant; pregnancy; serology.
Copyright © 2022 Bender, Lee, Cheng, Marentes Ruiz and Pannaraj.
Conflict of interest statement
PP has received research funding from AstraZeneca and Pfizer for unrelated studies, consultant fees from Sanofi-Pasteur and Seqirus, and speaker fees from Nestle Nutrition Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Persistence of COVID-19 Human Milk Antibodies After Maternal COVID-19 Vaccination: Systematic Review and Meta-Regression Analysis.Cureus. 2024 May 2;16(5):e59500. doi: 10.7759/cureus.59500. eCollection 2024 May. Cureus. 2024. PMID: 38826925 Free PMC article. Review.
-
Impact of maternal SARS-CoV-2 booster vaccination on blood and breastmilk antibodies.PLoS One. 2023 Jun 13;18(6):e0287103. doi: 10.1371/journal.pone.0287103. eCollection 2023. PLoS One. 2023. PMID: 37310982 Free PMC article.
-
Increase in SARS-CoV-2 RBD-Specific IgA and IgG Antibodies in Human Milk From Lactating Women Following the COVID-19 Booster Vaccination.J Hum Lact. 2023 Feb;39(1):51-58. doi: 10.1177/08903344221134631. Epub 2022 Nov 18. J Hum Lact. 2023. PMID: 36398916 Free PMC article.
-
Neutralizing Activity and SARS-CoV-2 Vaccine mRNA Persistence in Serum and Breastmilk After BNT162b2 Vaccination in Lactating Women.Front Immunol. 2022 Jan 11;12:783975. doi: 10.3389/fimmu.2021.783975. eCollection 2021. Front Immunol. 2022. PMID: 35087517 Free PMC article.
-
Are SARS-CoV-2 Antibodies Detectable in Human Milk After Vaccination Against COVID-19?J Pediatric Infect Dis Soc. 2022 Apr 30;11(4):126. doi: 10.1093/jpids/piac024. J Pediatric Infect Dis Soc. 2022. PMID: 35394545 Free PMC article.
Cited by
-
Breast Milk Conferred Immunity to Infants Against COVID-19.Cureus. 2023 Jul 18;15(7):e42075. doi: 10.7759/cureus.42075. eCollection 2023 Jul. Cureus. 2023. PMID: 37602015 Free PMC article. Review.
-
SARS-CoV-2 Vaccine Booster Elicits Robust Prolonged Maternal Antibody Responses and Passive Transfer Via The Placenta And Breastmilk.bioRxiv [Preprint]. 2022 Nov 29:2022.11.29.518385. doi: 10.1101/2022.11.29.518385. bioRxiv. 2022. Update in: Am J Obstet Gynecol MFM. 2023 Feb;5(2):100830. doi: 10.1016/j.ajogmf.2022.100830. PMID: 36482972 Free PMC article. Updated. Preprint.
-
Persistence of COVID-19 Human Milk Antibodies After Maternal COVID-19 Vaccination: Systematic Review and Meta-Regression Analysis.Cureus. 2024 May 2;16(5):e59500. doi: 10.7759/cureus.59500. eCollection 2024 May. Cureus. 2024. PMID: 38826925 Free PMC article. Review.
-
Safety and Efficacy of Coronavirus Disease 2019 (COVID-19) mRNA Vaccines During Lactation.Obstet Gynecol. 2023 Mar 1;141(3):483-491. doi: 10.1097/AOG.0000000000005093. Epub 2023 Jan 18. Obstet Gynecol. 2023. PMID: 36649326 Free PMC article. Review.
-
Impact of maternal SARS-CoV-2 booster vaccination on blood and breastmilk antibodies.PLoS One. 2023 Jun 13;18(6):e0287103. doi: 10.1371/journal.pone.0287103. eCollection 2023. PLoS One. 2023. PMID: 37310982 Free PMC article.
References
-
- Center for Disease Control Prevention . Care for Breastfeeding People: Interim Guidance on Breastfeeding and Breast Milk Feeds in the Context of Covid-19. (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-wom... (accessed December 29, 2021).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

