Mesenchymal Stem Cells-Derived Exosomes Ameliorate Ischemia/Reperfusion Induced Acute Kidney Injury in a Porcine Model
- PMID: 35686052
- PMCID: PMC9171021
- DOI: 10.3389/fcell.2022.899869
Mesenchymal Stem Cells-Derived Exosomes Ameliorate Ischemia/Reperfusion Induced Acute Kidney Injury in a Porcine Model
Abstract
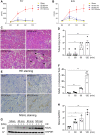
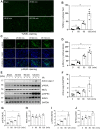
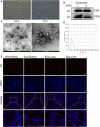
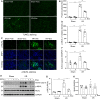
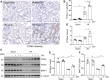
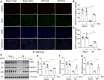
Exosomes are membrane-enclosed vesicles secreted by cells, containing a variety of biologically active ingredients including proteins, nucleic acids and lipids. In this study, we investigated the therapeutic effects of the exosomes and underlying mechanisms in a miniature pig model of ischemia/reperfusion-induced acute kidney injury (I/R-AKI). The exosomes were extracted from cultured human umbilical cord derived mesenchymal stem cells (hUC-MSCs) and infused into a miniature pig model of I/R AKI. Our results showed that 120 min of unilateral ischemia followed by reperfusion and contralateral nephrectomy resulted in renal dysfunction, severe kidney damage, apoptosis and necroptosis. Intravenous infusion of one dose of exosomes collected from about 4 × 108 hUC-MSCs significantly improved renal function and reduced apoptosis and necroptosis. Administration of hUC-MSC exosomes also reduced the expression of some pro-inflammatory cytokines/chemokines, decreased infiltration of macrophages to the injured kidneys and suppressed the phosphorylation of nuclear factor-κB and signal transducer and activator of transcription 3, two transcriptional factors related to inflammatory regulation. Moreover, hUC-MSC exosomes could promote proliferation of renal tubular cells, angiogenesis and upregulation of Klotho and Bone Morphogenetic Protein 7, two renoprotective molecules and vascular endothelial growth factor A and its receptor. Collectively, our results suggest that injection of hUC-MSC exosomes could ameliorate I/R-AKI and accelerate renal tubular cell repair and regeneration, and that hUC-MSC exosomes may be used as a potential biological therapy for Acute kidney injury patients.
Keywords: acute kidney injury; apoptosis; exosomes; human umbilical cord derived mesenchymal stem cells; ischemia/reperfusion; macrophages; necroptosis; transcriptional factors.
Copyright © 2022 Huang, Cao, Cui, Ma, Gao, Yu, Shen, Yang, Liu, Qiu, Cai and Zhuang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

