Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: A phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial
- PMID: 35686380
- PMCID: PMC10084286
- DOI: 10.1111/papr.13136
Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: A phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial
Erratum in
-
Corrigendum.Pain Pract. 2024 Sep;24(7):969-970. doi: 10.1111/papr.13376. Epub 2024 Apr 21. Pain Pract. 2024. PMID: 38644630 Free PMC article. No abstract available.
Abstract
Background: Celecoxib-tramadol co-crystal (CTC) is a first-in-class analgesic co-crystal of celecoxib and racemic tramadol with an improved pharmacologic profile, conferred by the co-crystal structure, compared with its active constituents administered alone/concomitantly.
Aim: We evaluated CTC in moderate-to-severe acute postoperative pain.
Materials and methods: This randomized, double-blind, factorial, active- and placebo-controlled phase 3 trial (NCT03108482) was conducted at 6 US clinical research centers. Adults with moderate-to-severe acute pain following bunionectomy with osteotomy were randomized to oral CTC (200 mg [112 mg celecoxib/88 mg rac-tramadol hydrochloride] every 12 h), tramadol (50 mg every 6 h), celecoxib (100 mg every 12 h), or placebo for 48 h. Patients, investigators, and personnel were blinded to assignment. The primary endpoint was the 0-48 h sum of pain intensity differences (SPID0-48) in all randomized patients. Pain intensity was assessed on a 0-10 numerical rating scale (NRS). Safety was analyzed in patients who received study medication. Funded by ESTEVE Pharmaceuticals.
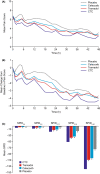
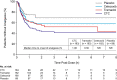
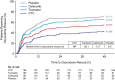
Results: In 2017 (March to November), 1323 patients were screened and 637 randomized to CTC (n = 184), tramadol (n = 183), celecoxib (n = 181), or placebo (n = 89). Mean baseline NRS was 6.7 in all active groups. CTC had a significantly greater effect on SPID0-48 (least-squares mean: -139.1 [95% confidence interval: -151.8, -126.5]) than tramadol (-109.1 [-121.7, -96.4]; p < 0.001), celecoxib (-103.7 [-116.4, -91.0]; p < 0.001), or placebo (-74.6 [-92.5, -56.6]; p < 0.001). Total treatment-emergent adverse events (TEAEs) were 358 for CTC and 394 for tramadol. Drug-related TEAEs occurred in 37.7% patients in the CTC group, compared with 48.6% in the tramadol group. There were no serious TEAEs/deaths.
Conclusion: CTC provided greater analgesia than comparable daily doses of tramadol and celecoxib, with similar tolerability to tramadol. CTC is approved in the United States.
Keywords: acute pain; analgesia; celecoxib; co-crystal; efficacy; pain; postoperative; postoperative pain; safety; tramadol.
© 2022 Esteve Pharmaceuticals S.A, Premier Research and The Authors. Pain Practice published by Wiley Periodicals LLC on behalf of World Institute of Pain.
Conflict of interest statement
This study was supported by ESTEVE Pharmaceuticals S.A. E.R. Viscusi reports consulting fees for ESTEVE Pharmaceuticals, Fresenius, Heron Therapeutics, Innocoll Pharmaceuticals, Merck, and Salix Pharmaceuticals. O. de Leon‐Casasola reports personal fees for advisory board membership for ESTEVE Pharmaceuticals during the conduct of the study, and for ESTEVE Pharmaceuticals, Stimgenix, and Medtronic outside the submitted work. J. Cebrecos, A. Morte, E. Ortiz, M. Sust, A. Vaqué, and N. Gascón are employees of ESTEVE Pharmaceuticals. A. Jacobs is an employee of Premier Research (Premier Research was paid commercial fees by ESTEVE Pharmaceuticals for work on the study). I. Gottlieb reports grants for ESTEVE Pharmaceuticals for participation as a principal investigator in the current study and personal fees for ESTEVE Pharmaceuticals for consulting projects. S. Daniels is an employee of Optimal Research (Optimal Research was paid commercial fees by ESTEVE Pharmaceuticals for work on the study). M.E. Kuss was an employee of Premier Research during the conduct of the study. S. Videla was an employee of ESTEVE Pharmaceuticals during the conduct of the study. C. Plata‐Salamán was an employee of ESTEVE Pharmaceuticals and has pending or issued patents relevant to CTC. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- FDA . US Food and Drug Administration. Prescribing information, SEGLENTIS. 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213426s000lbl.pdf. Accessed November 9, 2021.
-
- Gascon N, Almansa C, Merlos M, et al. Co‐crystal of tramadol‐celecoxib: preclinical and clinical evaluation of a novel analgesic. Expert Opin Investig Drugs. 2019;28:399–409. - PubMed
-
- Almansa C, Mercè R, Tesson N, et al. Co‐crystal of tramadol hydrochloride−celecoxib (CTC): a novel API − API co‐crystal for the treatment of pain. Cryst Growth Des. 2017;17:1884–92.
-
- Port A, Almansa C, Enrech R, et al. Differential solution behavior of the new API‐API co‐crystal of tramadol‐celecoxib (CTC) versus its constituents and their combination. Cryst Growth Des. 2019;19:3172–82.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

