Final safety and efficacy results from a 106 real-world patients registry with an ascites-mobilizing pump
- PMID: 35686702
- PMCID: PMC9543940
- DOI: 10.1111/liv.15337
Final safety and efficacy results from a 106 real-world patients registry with an ascites-mobilizing pump
Abstract
Background and aims: Patients with cirrhotic refractory ascites ineligible for transjugular intrahepatic shunt (TIPSS) have limited treatment options apart from repeated large volume paracentesis. The alfapump® is an implantable device mobilizing ascites from the peritoneal cavity to the bladder, from where it can be excreted. The aim of this observational cohort study was to prospectively investigate safety and efficacy of the device in a real-world cohort with cirrhotic refractory ascites and contraindications for TIPSS.
Methods: A total of 106 patients received an implant at 12 European centres and were followed up for up to 24 months. Complications, device deficiencies, frequency of paracentesis, clinical status and survival were recorded prospectively.
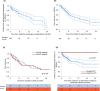
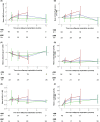
Results: Approximately half of the patients died on-study, about a quarter was withdrawn because of serious adverse events leading to explant, a sixth were withdrawn because of liver transplant or recovery, and nine completed follow-up. The most frequent causes of on-study death and complication-related explant were progression of liver disease and infection. The device reduced the requirement for large-volume paracentesis significantly, with more than half of patients not having required any post-implant. Survival benefits were not observed. Device-related reinterventions were predominantly caused by device deficiencies. A post-hoc comparison of the first 50 versus the last 50 patients enrolled revealed a decreased reintervention rate in the latter, mainly related to peritoneal catheter modifications.
Conclusions: The device reduced paracentesis frequency in a real-world setting. Technical complications were successfully decreased by optimization of management and device modification (NCT01532427).
Keywords: TIPSS; alfapump; ascites; cirrhosis; large-volume paracentesis.
© 2022 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
GS has received support for the present manuscript, travel and meeting attendance, served as a speaker and participated in Advisory Boards for Sequana Medical. TB has received grants or contracts with Abbvie, BMS, Gilead, Humedics, Intercept, MSD/Merck, Merz, Novartis and Sequana Medical, received consulting fees from Abbvie, Alexion, Bayer, Eisai, Gilead, GSK, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Roche, Sequana Medical and Shionogi and served as a speaker for MedUpdate GmbH and the abovementioned except GSK, Roche and Shionogi, He has received support for travel and meeting attendance from Abbvie, Gilead, Intercept and Janssen. LS has received lecture honoraria from and participated in Advisory Boards of Sequana Medical. SZ has served as a speaker and a consultant to Abbvie, Gilead, Intercept, Janssen, Merck/MSD. SMP has served as a consultant to Sequana Medical, AbbVie, Gilead, Cambwick and Intercept. FS has served as a speaker to Sequana Medical. CE has received research grants from Sequana Medical, the German Research Foundation (DFG) and the Berlin Institute of Health (BIH). AG has received consulting fees from Abbvie, Alexion, Bayer, BMS, CSL Behring, Eisai, Gilead, Intercept, Ipsen, Merz MSD, Novartis, Pfizer, Roche, Sanofi‐Aventis and Sequana Medical, received support for meeting attendance and travel from Abbvie, Gilead, Intercept and Merz and participated on a Data Safety Monitoring Board or Advisory Board for Novartis. CT is a biostatistician contracted by Sequana Medical. JC is an employee of and holds shares and stock options of Sequana Medical. ADG has received a research grant from Sequana Medical. FL, JB, VV and VB have nothing to disclose.
Figures

References
-
- Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30(7):937‐947. - PubMed
-
- Adebayo D, Neong SF, Wong F. Refractory ascites in liver cirrhosis. Am J Gastroenterol. 2019;114(1):40‐47. - PubMed
-
- Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta‐analysis of individual patient data. Gastroenterology. 2007;133(3):825‐834. - PubMed
-
- EASL . EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397‐417. - PubMed

