Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs
- PMID: 35686704
- PMCID: PMC9542194
- DOI: 10.1002/term.3328
Transplantation of encapsulated autologous olfactory ensheathing cell populations expressing chondroitinase for spinal cord injury: A safety and feasibility study in companion dogs
Abstract
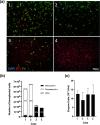
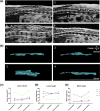
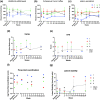
Spinal cord injury (SCI) can cause irreversible paralysis, with no regenerative treatment clinically available. Dogs with natural SCI present an established model and can facilitate translation of experimental findings in rodents to people. We conducted a prospective, single arm clinical safety study in companion dogs with chronic SCI to characterize the feasibility of intraspinal transplantation of hydrogel-encapsulated autologous mucosal olfactory ensheathing cell (mOEC) populations expressing chondroitinase ABC (chABC). mOECs and chABC are both promising therapies for SCI, and mOECs expressing chABC drive greater voluntary motor recovery than mOECs alone after SCI in rats. Canine mOECs encapsulated in collagen hydrogel can be matched in stiffness to canine SCI. Four dogs with complete and chronic loss of function caudal to a thoraco-lumbar lesion were recruited. After baseline measures, olfactory mucosal biopsy was performed and autologous mOECs cultured and transduced to express chABC, then hydrogel-encapsulated and percutaneously injected into the spinal cord. Dogs were monitored for 6 months with repeat clinical examinations, spinal MRI, kinematic gait and von Frey assessment. No adverse effects or significant changes on neurological examination were detected. MRI revealed large and variable lesions, with no spinal cord compression or ischemia visible after hydrogel transplantation. Owners reported increased pelvic-limb reflexes with one dog able to take 2-3 unsupported steps, but gait-scoring and kinematic analysis showed no significant improvements. This novel combination approach to regeneration after SCI is therefore feasible and safe in paraplegic dogs in a clinical setting. A randomised-controlled trial in this translational model is proposed to test efficacy.
Keywords: canine translational model; cell therapy / transplantation; chondroitinase ABC; hydrogel encapsulation; neurology; neuroscience; olfactory ensheathing cells; spinal cord injury; spontaneous animal model.
© 2022 The Authors. Journal of Tissue Engineering and Regenerative Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures
References
-
- Bakker, M. H. , Tseng, C. C. S. , Keizer, H. M. , Seevinck, P. R. , Janssen, H. M. , Van Slochteren, F. J. , Chamuleau, S. A. J. , & Dankers, P. Y. W. (2018). MRI visualization of injectable ureidopyrimidinone hydrogelators by supramolecular contrast agent labeling. Advanced Healthcare Materials, 7(11), 1701139. 10.1002/adhm.201701139 - DOI - PubMed
-
- Fawcett, J. W. , Curt, A. , Steeves, J. D. , Coleman, W. P. , Tuszynski, M. H. , Lammertse, D. , Lammertse, D. , Blight, A. R. , Dietz, V. , Ditunno, J. , Dobkin, B. H. , Havton, L. A. , Ellaway, P. H. , Fehlings, M. G. , PrivAt, A. , GRossman, R. , Guest, J. D. , Kleitma, N. N. , NakaMura, M. , …, & Short, D. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord, 45(3), 190–205. 10.1038/sj.sc.3102007 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

