Borylation Directed Borylation of Indoles Using Pyrazabole Electrophiles: A One-Pot Route to C7-Borylated-Indolines
- PMID: 35686751
- PMCID: PMC9401042
- DOI: 10.1002/anie.202206230
Borylation Directed Borylation of Indoles Using Pyrazabole Electrophiles: A One-Pot Route to C7-Borylated-Indolines
Abstract
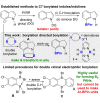
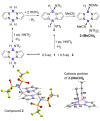
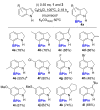
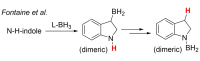
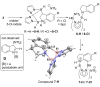
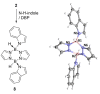
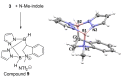
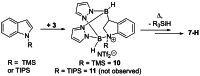
Pyrazabole (1) is a readily accessible diboron compound that can be transformed into ditopic electrophiles. In 1 (and derivatives), the B⋅⋅⋅B separation is ca. 3 Å, appropriate for one boron centre bonding to N and one to the C7 of indoles and indolines. This suitable B⋅⋅⋅B separation enables double E-H (E=N/C) functionalisation of indoles and indolines. Specifically, the activation of 1 with HNTf2 generates an electrophile that transforms N-H indoles and indolines into N/C7-diborylated indolines, with N-H borylation directing subsequent C7-H borylation. Indole reduction to indoline occurs before C-H borylation and our studies indicate this proceeds via hydroboration-C3-protodeboronation to produce an intermediate that then undergoes C7 borylation. The borylated products can be converted in situ into C7-BPin-N-H-indolines. Overall, this represents a transient directed C-H borylation to form useful C7-BPin-indolines.
Keywords: Boranes; C−H Borylation; Electrophilic Substitution; Indoles; Transient Directing Group.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

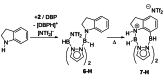
References
-
- Boronic Acids: Preparation and Applications (Ed.: Hall D.), Wiley-VCH, Weinheim, 2011.
-
- None
-
- Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F., Chem. Rev. 2010, 110, 890; - PubMed
-
- Xu L., Wang G., Zhang S., Wang H., Wang L., Liu L., Jiao J., Li P., Tetrahedron 2017, 73, 7123;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

