Whole-genome sequencing analysis and protocol for RNA interference of the endoparasitoid wasp Asobara japonica
- PMID: 35686927
- PMCID: PMC9233498
- DOI: 10.1093/dnares/dsac019
Whole-genome sequencing analysis and protocol for RNA interference of the endoparasitoid wasp Asobara japonica
Abstract
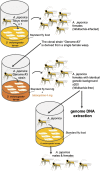
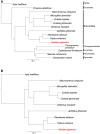
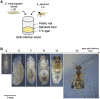
Asobara japonica is an endoparasitic wasp that parasitizes Drosophila flies. It synthesizes various toxic components in the venom gland and injects them into host larvae during oviposition. To identify and characterize these toxic components for enabling parasitism, we performed the whole-genome sequencing (WGS) and devised a protocol for RNA interference (RNAi) with A. japonica. Because it has a parthenogenetic lineage due to Wolbachia infection, we generated a clonal strain from a single wasp to obtain highly homogenous genomic DNA. The WGS analysis revealed that the estimated genome size was 322 Mb with a heterozygosity of 0.132%. We also performed RNA-seq analyses for gene annotation. Based on the qualified WGS platform, we cloned ebony-Aj, which encodes the enzyme N-β-alanyl dopamine synthetase, which is involved in melanin production. The microinjection of double-stranded RNA (dsRNA) targeting ebony-Aj led to body colour changes in adult wasps, phenocopying ebony-Dm mutants. Furthermore, we identified putative venom genes as a target of RNAi, confirming that dsRNA injection-based RNAi specifically suppressed the expression of the target gene in wasp adults. Taken together, our results provide a powerful genetic toolkit for studying the molecular mechanisms of parasitism.
Keywords: Asobara japonica; RNA interference; endoparasitoid wasp; parasitism; whole-genome sequencing.
© The Author(s) 2022. Published by Oxford University Press on behalf of Kazusa DNA Research Institute.
Figures

Similar articles
-
Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism.Sci Adv. 2025 Jan 31;11(5):eadq8771. doi: 10.1126/sciadv.adq8771. Epub 2025 Jan 29. Sci Adv. 2025. PMID: 39879297 Free PMC article.
-
Development of RNAi in a Drosophila endoparasitoid wasp and demonstration of its efficiency in impairing venom protein production.J Insect Physiol. 2014 Apr;63:56-61. doi: 10.1016/j.jinsphys.2014.02.011. Epub 2014 Mar 4. J Insect Physiol. 2014. PMID: 24607638
-
Venom components of Asobara japonica impair cellular immune responses of host Drosophila melanogaster.Arch Insect Biochem Physiol. 2013 Jun;83(2):86-100. doi: 10.1002/arch.21093. Epub 2013 Apr 19. Arch Insect Biochem Physiol. 2013. PMID: 23606512
-
Integrative approach reveals composition of endoparasitoid wasp venoms.PLoS One. 2013 May 23;8(5):e64125. doi: 10.1371/journal.pone.0064125. Print 2013. PLoS One. 2013. PMID: 23717546 Free PMC article.
-
Components of Asobara venoms and their effects on hosts.Adv Parasitol. 2009;70:217-32. doi: 10.1016/S0065-308X(09)70008-9. Adv Parasitol. 2009. PMID: 19773072 Review.
Cited by
-
Development of a Stage- and Species-Specific RNAi System for Molecular Insights in Trichogramma Wasps.Insects. 2025 Jun 27;16(7):673. doi: 10.3390/insects16070673. Insects. 2025. PMID: 40725304 Free PMC article.
-
Host and venom evolution in parasitoid wasps: does independently adapting to the same host shape the evolution of the venom gland transcriptome?BMC Biol. 2024 Aug 15;22(1):174. doi: 10.1186/s12915-024-01974-2. BMC Biol. 2024. PMID: 39148049 Free PMC article.
-
Construction of a Banker Plant System via the Host Switch Trait of a Natural Enemy Aenasius bambawalei.Life (Basel). 2023 Oct 25;13(11):2115. doi: 10.3390/life13112115. Life (Basel). 2023. PMID: 38004255 Free PMC article.
-
Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism.Sci Adv. 2025 Jan 31;11(5):eadq8771. doi: 10.1126/sciadv.adq8771. Epub 2025 Jan 29. Sci Adv. 2025. PMID: 39879297 Free PMC article.
References
-
- Pennacchio F., Strand M.R.. 2006, Evolution of developmental strategies in parasitic hymenoptera, Annu. Rev. Entomol., 51, 233–58. - PubMed
-
- Quicke D.L. (ed.) 1997, Parasitic Wasps. Springer, Germany, 221–55.
-
- Quicke D.L. 2015, The Braconid and Ichneumonid Parasitoid Wasps. Wiley-Blackwell, USA.
-
- Yang L., Qiu L.M., Fang Q., Stanley D.W., Ye G.Y.. 2021, Cellular and humoral immune interactions between Drosophila and its parasitoids, Insect Sci., 28, 1208–27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

