Secretory Cells Are the Primary Source of pIgR in Small Airways
- PMID: 35687143
- PMCID: PMC9447142
- DOI: 10.1165/rcmb.2021-0548OC
Secretory Cells Are the Primary Source of pIgR in Small Airways
Abstract
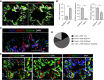
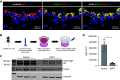
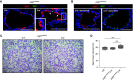
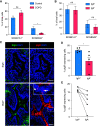
Loss of secretory IgA (SIgA) is common in chronic obstructive pulmonary disease (COPD) small airways and likely contributes to disease progression. We hypothesized that loss of SIgA results from reduced expression of pIgR (polymeric immunoglobulin receptor), a chaperone protein needed for SIgA transcytosis, in the COPD small airway epithelium. pIgR-expressing cells were defined and quantified at single-cell resolution in human airways using RNA in situ hybridization, immunostaining, and single-cell RNA sequencing. Complementary studies in mice used immunostaining, primary murine tracheal epithelial cell culture, and transgenic mice with secretory or ciliated cell-specific knockout of pIgR. SIgA degradation by human neutrophil elastase or secreted bacterial proteases from nontypeable Haemophilus influenzae was evaluated in vitro. We found that secretory cells are the predominant cell type responsible for pIgR expression in human and murine airways. Loss of SIgA in small airways was not associated with a reduction in secretory cells but rather a reduction in pIgR protein expression despite intact PIGR mRNA expression. Neutrophil elastase and nontypeable H. influenzae-secreted proteases are both capable of degrading SIgA in vitro and may also contribute to a deficient SIgA immunobarrier in COPD. Loss of the SIgA immunobarrier in small airways of patients with severe COPD is complex and likely results from both pIgR-dependent defects in IgA transcytosis and SIgA degradation.
Keywords: airway epithelium; chronic obstructive pulmonary disease; secretory IgA; secretory cells.
Figures

Comment in
-
Secretory Cells: New Players in Small Airway Mucosal Immunity?Am J Respir Cell Mol Biol. 2022 Sep;67(3):269-270. doi: 10.1165/rcmb.2022-0210ED. Am J Respir Cell Mol Biol. 2022. PMID: 35704450 Free PMC article. No abstract available.
References
-
- Muir DC. Bulk flow and diffusion in the airways of the lung. Br J Dis Chest . 1966;60:169–176. - PubMed
-
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev . 2000;173:27–38. - PubMed
-
- Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev . 2005;206:83–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

