Catalytic Enantioselective Entry to Triflones Featuring a Quaternary Stereocenter
- PMID: 35687515
- PMCID: PMC9490835
- DOI: 10.1021/acs.orglett.2c01589
Catalytic Enantioselective Entry to Triflones Featuring a Quaternary Stereocenter
Abstract
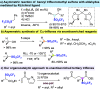
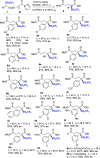
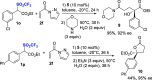
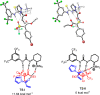
A highly enantioselective one-pot synthesis of functionalized triflones, bearing a quaternary stereocenter, has been developed, exploiting the Michael reaction of α-(trifluoromethylsulfonyl) aryl acetic acid esters with N-acryloyl-1H-pyrazole catalyzed by commercially available Takemoto's catalyst, followed by nucleophilic acyl substitution with alcohols. Preliminary investigations highlighted the attractive potential of the triflinate anion as the leaving group for stereocontrolled postfunctionalizations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Scott J. P.; Oliver S. F.; Brands K. M. J.; Brewer S. E.; Davies A. J.; Gibb A. D.; Hands D.; Keen S. P.; Sheen F. J.; Reamer R. A.; Wilson R. D.; Dolling U.-H. Practical Asymmetric Synthesis of a γ-Secretase Inhibitor Exploiting Substrate-Controlled Intramolecular Nitrile Oxide Olefin Cycloaddition. J. Org. Chem. 2006, 71, 3086–3092. 10.1021/jo060033i. - DOI - PubMed
-
- Velázquez F.; Sannigrahi M.; Bennett F.; Lovey R. G.; Arasappan A.; Bogen S.; Nair L.; Venkatraman S.; Blackman M.; Hendrata S.; Huang Y.; Huelgas R.; Pinto P.; Cheng K.-C.; Tong X.; McPhail A. T.; Njoroge F. G. Cyclic Sulfones as Novel P3-Caps for Hepatitis C Virus NS3/4A (HCV NS3/4A) Protease Inhibitors: Synthesis and Evaluation of Inhibitors with Improved Potency and Pharmacokinetic Profiles. J. Med. Chem. 2010, 53, 3075–3085. 10.1021/jm9016027. - DOI - PubMed
-
- Trost B. M. Chemical Chameleons. Organosulfones as Synthetic Building Blocks. Bull. Chem. Soc. Jpn. 1988, 61, 107–124. 10.1246/bcsj.61.107. - DOI
- El-Awa A.; Noshi M. N.; du Jourdin X. M.; Fuchs P. L. Evolving Organic Synthesis Fostered by the Pluripotent Phenylsulfone. Moiety. Chem. Rev. 2009, 109, 2315–2349. 10.1021/cr800309r. - DOI - PubMed
- Xu X. H.; Matsuzaki K.; Shibata N. Synthetic Methods for Compounds Having CF3-S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. 10.1021/cr500193b. - DOI - PubMed

