Carba Analogues of Flupirtine and Retigabine with Improved Oxidation Resistance and Reduced Risk of Quinoid Metabolite Formation
- PMID: 35687532
- PMCID: PMC9541272
- DOI: 10.1002/cmdc.202200262
Carba Analogues of Flupirtine and Retigabine with Improved Oxidation Resistance and Reduced Risk of Quinoid Metabolite Formation
Abstract
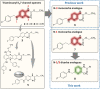
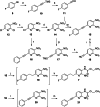
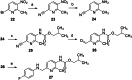
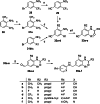
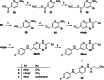
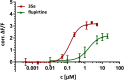
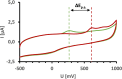
The KV 7 potassium channel openers flupirtine and retigabine have been valuable options in the therapy of pain and epilepsy. However, as a result of adverse reactions, both drugs are currently no longer in therapeutic use. The flupirtine-induced liver injury and the retigabine linked tissue discolouration do not appear related at first glance; nevertheless, both events can be attributed to the triaminoaryl scaffold, which is affected by oxidation leading to elusive reactive quinone diimine or azaquinone diimine metabolites. Since the mechanism of action, i. e. KV 7 channel opening, seems not to be involved in toxicity, this study aimed to further develop safer replacements for flupirtine and retigabine. In a ligand-based design strategy, replacing amino substituents of the triaminoaryl core with alkyl substituents led to carba analogues with improved oxidation resistance and negligible risk of quinoid metabolite formation. In addition to these improved safety features, some of the novel analogues exhibited significantly improved KV 7.2/3 channel opening activity, indicated by an up to 13-fold increase in potency and an efficacy of up to 176 % compared to flupirtine, thus being attractive candidates for further development.
Keywords: KV7; drug design; flupirtine; ion channels; retigabine.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Sulfide Analogues of Flupirtine and Retigabine with Nanomolar KV 7.2/KV 7.3 Channel Opening Activity.ChemMedChem. 2019 May 6;14(9):952-964. doi: 10.1002/cmdc.201900112. Epub 2019 Apr 2. ChemMedChem. 2019. PMID: 30861620
-
Replacing the oxidation-sensitive triaminoaryl chemotype of problematic KV 7 channel openers: Exploration of a nicotinamide scaffold.Arch Pharm (Weinheim). 2023 Feb;356(2):e2200473. doi: 10.1002/ardp.202200473. Epub 2022 Nov 17. Arch Pharm (Weinheim). 2023. PMID: 36395379
-
Antinociceptive Efficacy of Retigabine and Flupirtine for Gout Arthritis Pain.Pharmacology. 2020;105(7-8):471-476. doi: 10.1159/000505934. Epub 2020 Feb 14. Pharmacology. 2020. PMID: 32062659
-
The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy.Epilepsia. 2012 Mar;53(3):412-24. doi: 10.1111/j.1528-1167.2011.03365.x. Epub 2012 Jan 5. Epilepsia. 2012. PMID: 22220513 Review.
-
Flupirtine, a re-discovered drug, revisited.Inflamm Res. 2013 Mar;62(3):251-8. doi: 10.1007/s00011-013-0592-5. Epub 2013 Jan 16. Inflamm Res. 2013. PMID: 23322112 Review.
Cited by
-
Green electrosynthesis of drug metabolites.Toxicol Res (Camb). 2023 Mar 7;12(2):150-177. doi: 10.1093/toxres/tfad009. eCollection 2023 Apr. Toxicol Res (Camb). 2023. PMID: 37125339 Free PMC article. Review.
-
Targeting Kv7 Potassium Channels for Epilepsy.CNS Drugs. 2025 Mar;39(3):263-288. doi: 10.1007/s40263-024-01155-3. Epub 2025 Jan 24. CNS Drugs. 2025. PMID: 39853501 Free PMC article. Review.
-
Three-Step Synthesis of the Antiepileptic Drug Candidate Pynegabine.Molecules. 2023 Jun 21;28(13):4888. doi: 10.3390/molecules28134888. Molecules. 2023. PMID: 37446549 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

