Mucosal immunity of mannose-modified chitosan microspheres loaded with the nontyepable Haemophilus influenzae outer membrane protein P6 in BALB/c mice
- PMID: 35687548
- PMCID: PMC9187061
- DOI: 10.1371/journal.pone.0269153
Mucosal immunity of mannose-modified chitosan microspheres loaded with the nontyepable Haemophilus influenzae outer membrane protein P6 in BALB/c mice
Abstract
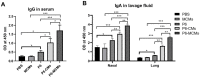
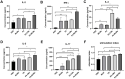
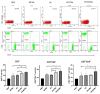
Nontypeable Haemophilus influenzae (NTHi) is a common opportunistic pathogen that colonizes the nasopharynx. NTHi infections result in enormous global morbidity in two clinical settings: otitis media in children and acute exacerbation of chronic obstructive pulmonary disease (COPD) in adults. Thus, there is an urgent need to design and develop effective vaccines to prevent morbidity and reduce antibiotic use. The NTHi outer membrane protein P6, a potential vaccine candidate, is highly conserved and effectively induces protective immunity. Here, to enhance mucosal immune responses, P6-loaded mannose-modified chitosan (MC) microspheres (P6-MCMs) were developed for mucosal delivery. MC (18.75%) was synthesized by the reductive amination reaction method using sodium cyanoborohydride (NaBH3CN), and P6-MCMs with an average size of 590.4±16.2 nm were successfully prepared via the tripolyphosphate (TPP) ionotropic gelation process. After intranasal immunization with P6-MCMs, evaluation of humoral immune responses indicated that P6-MCMs enhance both systemic and mucosal immune responses. Evaluation of cellular immune responses indicated that P6-MCMs enhance cellular immunity and trigger a mixed Th1/Th2-type immune response. Importantly, P6-MCMs also trigger a Th17-type immune response. They are effective in promoting lymphocyte proliferation and differentiation without toxicity in vitro. The results also demonstrate that P6-MCMs can effectively induce MHC class I- and II-restricted cross-presentation, promoting CD4+-mediated Th immune responses and CD8+-mediated cytotoxic T lymphocyte (CTL) immune responses. Evaluation of protective immunity indicated that immunization with P6-MCMs can reduce inflammation in the nasal mucosa and the lung and prevent NTHi infection. In conclusion, MCMs are a promising adjuvant-delivery system for vaccines against NTHi.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Induction of Mucosal IgA-Mediated Protective Immunity Against Nontypeable Haemophilus influenzae Infection by a Cationic Nanogel-Based P6 Nasal Vaccine.Front Immunol. 2022 Jul 6;13:819859. doi: 10.3389/fimmu.2022.819859. eCollection 2022. Front Immunol. 2022. PMID: 35874779 Free PMC article.
-
Nasal immunization with plasmid DNA encoding P6 protein and immunostimulatory complexes elicits nontypeable Haemophilus influenzae-specific long-term mucosal immune responses in the nasopharynx.Vaccine. 2011 Feb 24;29(10):1881-90. doi: 10.1016/j.vaccine.2010.12.129. Epub 2011 Jan 13. Vaccine. 2011. PMID: 21237276
-
Th17 cells contribute to nontypeable Haemophilus influenzae-specific protective immunity induced by nasal vaccination with P6 outer membrane protein and α-galactosylceramide.Microbiol Immunol. 2011 Aug;55(8):574-81. doi: 10.1111/j.1348-0421.2011.00352.x. Microbiol Immunol. 2011. PMID: 21605159
-
Developing a vaccine to prevent otitis media caused by nontypeable Haemophilus influenzae.Expert Rev Vaccines. 2016 Jul;15(7):863-78. doi: 10.1586/14760584.2016.1156539. Epub 2016 Mar 17. Expert Rev Vaccines. 2016. PMID: 26894630 Review.
-
Vaccines for Nontypeable Haemophilus influenzae: the Future Is Now.Clin Vaccine Immunol. 2015 May;22(5):459-66. doi: 10.1128/CVI.00089-15. Epub 2015 Mar 18. Clin Vaccine Immunol. 2015. PMID: 25787137 Free PMC article. Review.
Cited by
-
Natural Polymeric Composites Derived from Animals, Plants, and Microbes for Vaccine Delivery and Adjuvant Applications: A Review.Gels. 2023 Mar 15;9(3):227. doi: 10.3390/gels9030227. Gels. 2023. PMID: 36975676 Free PMC article. Review.
-
Chitosan-based formulations for therapeutic applications. A recent overview.J Biomed Sci. 2025 Jul 8;32(1):62. doi: 10.1186/s12929-025-01161-7. J Biomed Sci. 2025. PMID: 40629425 Free PMC article. Review.
-
Applications of Chitosan in Prevention and Treatment Strategies of Infectious Diseases.Pharmaceutics. 2024 Sep 13;16(9):1201. doi: 10.3390/pharmaceutics16091201. Pharmaceutics. 2024. PMID: 39339237 Free PMC article. Review.
-
Targeting Respiratory Viruses: The Efficacy of Intranasal mRNA Vaccination in Generating Protective Mucosal and Systemic Immunity Against Influenza A (H1N1).Influenza Other Respir Viruses. 2025 Mar;19(3):e70093. doi: 10.1111/irv.70093. Influenza Other Respir Viruses. 2025. PMID: 40127967 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

