Bovine colostrum-derived antibodies against SARS-CoV-2 show great potential to serve as prophylactic agents
- PMID: 35687549
- PMCID: PMC9187060
- DOI: 10.1371/journal.pone.0268806
Bovine colostrum-derived antibodies against SARS-CoV-2 show great potential to serve as prophylactic agents
Abstract
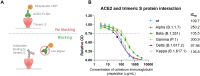
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to impose a serious burden on health systems globally. Despite worldwide vaccination, social distancing and wearing masks, the spread of the virus is ongoing. One of the mechanisms by which neutralizing antibodies (NAbs) block virus entry into cells encompasses interaction inhibition between the cell surface receptor angiotensin-converting enzyme 2 (ACE2) and the spike (S) protein of SARS-CoV-2. SARS-CoV-2-specific NAb development can be induced in the blood of cattle. Pregnant cows produce NAbs upon immunization, and antibodies move into the colostrum immediately before calving. Here, we immunized cows with SARS-CoV-2 S1 receptor binding domain (RBD) protein in proper adjuvant solutions, followed by one boost with SARS-CoV-2 trimeric S protein and purified immunoglobulins from colostrum. We demonstrate that this preparation indeed blocks the interaction between the trimeric S protein and ACE2 in different in vitro assays. Moreover, we describe the formulation of purified immunoglobulin preparation into a nasal spray. When administered to human subjects, the formulation persisted on the nasal mucosa for at least 4 hours, as determined by a clinical study. Therefore, we are presenting a solution that shows great potential to serve as a prophylactic agent against SARS-CoV-2 infection as an additional measure to vaccination and wearing masks. Moreover, our technology allows for rapid and versatile adaptation for preparing prophylactic treatments against other diseases using the defined characteristics of antibody movement into the colostrum.
Conflict of interest statement
The use of bovine colostrum as a prophylactic agent against SARS-CoV-2 has been patented (US patent application no 63/160,833) by Mario Plaas, K. Kogermann, E. Žusinaite, T. Tiirats, B. Aasmäe, A. Kavak, V. Poikalainen, L. Lepasalu, S. Piiskop, S. Rom, R. Oltjer, K. Kangro, E. Sankovski, J. M. Gerhold, R. Pert, A. Männik, A. Planken, A. Tover, M. Kurašin, M. Ustav and M. Ustav Jr.
Figures





Similar articles
-
Adjuvant-driven antibody response to use cows as biofactories of anti-SARS-CoV-2 neutralizing antibodies in colostrum.J Dairy Sci. 2025 Apr;108(4):4079-4088. doi: 10.3168/jds.2024-25930. Epub 2025 Jan 30. J Dairy Sci. 2025. PMID: 39892600
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
Epitope Classification and RBD Binding Properties of Neutralizing Antibodies Against SARS-CoV-2 Variants of Concern.Front Immunol. 2021 Jun 4;12:691715. doi: 10.3389/fimmu.2021.691715. eCollection 2021. Front Immunol. 2021. PMID: 34149735 Free PMC article.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
A Structural Landscape of Neutralizing Antibodies Against SARS-CoV-2 Receptor Binding Domain.Front Immunol. 2021 Apr 28;12:647934. doi: 10.3389/fimmu.2021.647934. eCollection 2021. Front Immunol. 2021. PMID: 33995366 Free PMC article. Review.
Cited by
-
Antiviral Immunoglobulins of Chicken Egg Yolk for Potential Prevention of SARS-CoV-2 Infection.Viruses. 2022 Sep 26;14(10):2121. doi: 10.3390/v14102121. Viruses. 2022. PMID: 36298676 Free PMC article.
-
Transchromosomic bovines-derived broadly neutralizing antibodies as potent biotherapeutics to counter important emerging viral pathogens with a special focus on SARS-CoV-2, MERS-CoV, Ebola, Zika, HIV-1, and influenza A virus.J Med Virol. 2022 Oct;94(10):4599-4610. doi: 10.1002/jmv.27907. Epub 2022 Jun 11. J Med Virol. 2022. PMID: 35655326 Free PMC article. Review.
-
Hyperimmune immunoglobulin for people with COVID-19.Cochrane Database Syst Rev. 2023 Jan 26;1(1):CD015167. doi: 10.1002/14651858.CD015167.pub2. Cochrane Database Syst Rev. 2023. PMID: 36700518 Free PMC article.
-
SARS CoV-2 infections in animals, two years into the pandemic.Arch Virol. 2022 Dec;167(12):2503-2517. doi: 10.1007/s00705-022-05609-1. Epub 2022 Oct 7. Arch Virol. 2022. PMID: 36207554 Free PMC article. Review.
-
Bovine colostrum and its potential contributions for treatment and prevention of COVID-19.Front Immunol. 2023 Oct 16;14:1214514. doi: 10.3389/fimmu.2023.1214514. eCollection 2023. Front Immunol. 2023. PMID: 37908368 Free PMC article. Review.
References
-
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al.. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. Nature Research; 2020. pp. 536–544. doi: 10.1038/s41564-020-0695-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

