Effectiveness of a Second Dose of an mRNA Vaccine Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Infection in Individuals Previously Infected by Other Variants
- PMID: 35687580
- PMCID: PMC9214148
- DOI: 10.1093/cid/ciac429
Effectiveness of a Second Dose of an mRNA Vaccine Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Infection in Individuals Previously Infected by Other Variants
Abstract
Background: Single-dose vaccination was widely recommended in the pre-Omicron era for persons with previous SARS-CoV-2 infection. The effectiveness of a second vaccine dose in this group in the Omicron era is unknown.
Methods: We linked nationwide population registries in Spain to identify community-dwelling individuals aged 18-64, with a positive SARS-CoV-2 test before single-dose mRNA vaccination (mRNA-1273 or BNT162b2). Every day between 3 January and 6 February 2022 we matched 1:1 individuals receiving a second mRNA vaccine dose and controls on sex, age, province, first dose type and time, month of primary infection, and number of previous tests. We then estimated Kaplan-Meier risks of confirmed SARS-CoV-2 reinfection. We performed a similar analysis in a Delta-dominant period, between 19 July and 30 November 2021.
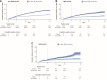
Results: In the Omicron period, estimated effectiveness (95% CI) of a second dose was 62.2% (58.2-66.4%) 7-34 days after administration, similar across groups defined by age, sex, type of first vaccine, and time since the first dose. Estimated effectiveness was 65.4% (61.1-69.9%) for mRNA-1273 and 52.0% (41.8-63.1%) for BNT162b2. Estimated effectiveness was 78.5% (67.4-89.9%), 66.1% (54.9-77.5%), and 60.2% (55.5-64.8%) when primary infection had occurred in the Delta, Alpha, and pre-Alpha periods, respectively. In the Delta period, the estimated effectiveness of a second dose was 8.8% (-55.3% to 81.1%).
Conclusions: Our results suggest that, over 1 month after administration, a second dose of mRNA vaccine increases protection against SARS-CoV-2 reinfection with the Omicron variant among individuals with single-dose vaccination and previously infected with another variant.
Keywords: COVID-19; Omicron; SARS-CoV-2; effectiveness; vaccines.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. A. H. reports consulting fees from ProPublica for work as a Data Science Advisor and from Cytel for work as a Methodological consultant. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Grupo de Trabajo Técnico de Vacunación COVID-19, de la Ponencia de Programa y Registro de Vacunaciones. Estrategia de vacunación frente a COVID 19 en España [Internet] . Available at:http://www.mscbs.es/profesionales/saludPublica/ccayes/alertasActual/nCov.... Accessed 8 March 2022.
-
- European Centre for Disease Prevention and Control (ECDC) . Technical report. Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. 31 January 2022 [Internet]. Available at:https://www.ecdc.europa.eu/sites/default/files/documents/Overview-of-COV.... Accessed 8 March 2022.
-
- Mahallawi WH, Fakher MH, Alsarani MA, Aljohani RH, Al-Mutabgani SA, Ibrahim NA. A single dose of SARS-CoV-2 vaccine primes a strong humoral immune response in COVID-19-recovered patients. Viral Immunol 2022;35:122–8. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

