A qPCR-duplex assay for sex determination in ancient DNA
- PMID: 35687599
- PMCID: PMC9187067
- DOI: 10.1371/journal.pone.0269913
A qPCR-duplex assay for sex determination in ancient DNA
Abstract
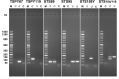
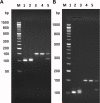
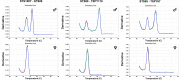
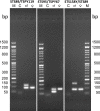
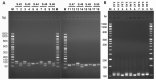
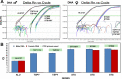
Molecular biology techniques are increasingly being used in sex identification of skeletal remains when traditional anthropometric analyzes are not successful in identifying sex of remains that are incomplete, fragmented and /or of immature individuals. In the present work, we investigated the possibility of determining sex by using the qPCR-duplex method for both ancient and modern DNA samples. This method involves the co-amplification of two genes in a single reaction system and the subsequent analysis of the fusion curves; the gene sequences used for the construction of suitable primers are those of steroid sulfatase (STS) and testis specific protein Y-linked 1 (TSPY) genes which turned out to be two sensitive markers as they have a detection limit of 60 pg and 20 pg respectively on modern DNA. The validity of the method was verified on modern DNA in which gender was identified in all the samples with 100% accuracy; thus, allowing for the same results as the classic method with amelogenin, but in a faster and more immediate way, as it allows for sex determination solely by analyzing the denaturation curves without having to perform an electrophoretic run. The proposed molecular technique proves to be sensitive and precise even on degraded DNA, in fact on 9 archaeological finds dating from the VII-XII century in which sex had been identified through anthropometric analysis, it confirmed the sex of 8 out of 9 finds correctly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rogers T., Saunders S. Accuracy of sex determination using morphological traits of the human pelvis. J Forensic Sci. 1994; 39(4):1047–56. . - PubMed
-
- Albanese J. A metric method for sex determination using the hipbone and the femur. J Forensic Sci 2003; 48(2):263–73. . - PubMed
-
- Francesquini Júnior L., Francesquini M.A., De La Cruz B.M., Pereira S.D., Ambrosano G.M., Barbosa C.M., et al.. Identification of sex using cranial base measurements. J Forensic Odontostomatol. 2007; 25(1):7–11. . - PubMed
-
- Daskalaki E., Anderung C., Humphrey L., Götherström A. Further developments in molecular sex assignment: a blind test of 18th and 19th century human skeletons. Journal of Archaeological Science. 2011; 38(6): 1326–1330. 10.1016/j.jas.2011.01.009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

